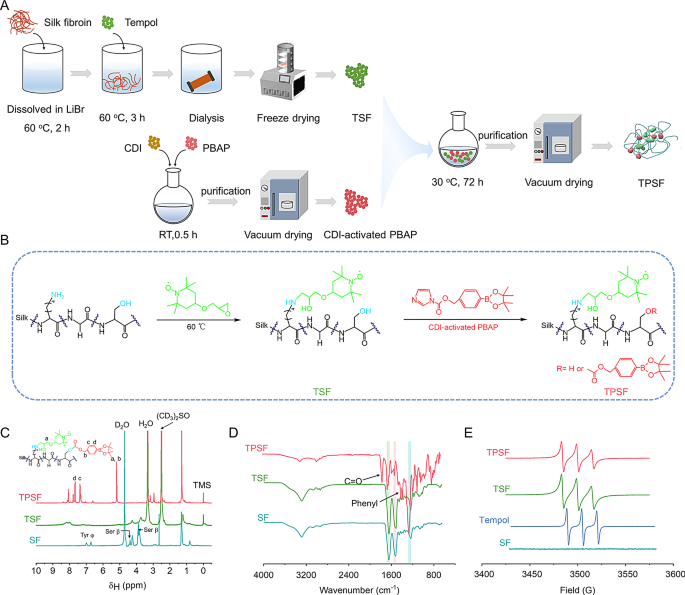
Synthesis and characterization of bioactive TPSF materials with ROS-scavenging capability
Tpl is a SOD-mimetic agent that may successfully neutralize superoxide anions and oxygen radicals [65]. In the meantime the PBAP unit is ready to stoichiometrically inactivate H2O2 [41]. Thus, we designed a silk-based ROS scavenger, outlined as TPSF, which was concurrently chemically modified with each Tpl and PBAP models. We hypothesize that nanomaterials based mostly on TPSF might function candidates for focused remedy of acute inflammatory illnesses by restoring the redox stability within the pathological microenvironment. The artificial strategy of TPSF is illustrated in Fig. 1A and B. Particularly, TPSF was synthesized through Tpl substitution of the first amines of SF in an alkaline setting, adopted by modification with CDI-activated PBAP on the hydroxyl teams of SF. In keeping with a beforehand reported research [59], Tpl-conjugated SF (TSF) supplies have been fabricated with a last Tpl focus of 282 mmol/L. For PBAP modification, the mass ratios of TSF to CDI-PBAP and the response time have been decided based mostly on H2O2 scavenging effectivity and 1H NMR spectra (Fig. S1A, B), leading to a 1:8 ratio for TPSF synthesis over a 72 h response interval. The 1H NMR spectra of TPSF indicated the attribute indicators at about 7.4 and seven.7 ppm, which have been assigned to the phenyl teams of PBAP (Fig. 1C). Of notice, the proton indicators at roughly 4.0, 4.4, 6.7, and seven.0 ppm have been considerably diminished and even disappeared after modification with the energetic models, suggesting that the energetic models have been covalently grafted onto the serine and tyrosine residues of SF. In addition to, the TPSF confirmed severely attenuated absorption at round 3300 cm− 1 within the Fourier-transform infrared (FT-IR) spectrum, which could possibly be attributed to the substitution of hydroxyl teams on SF (Fig. 1D). Additionally, new indicators noticed at roughly 1750 cm− 1 and 1600 –1450 cm− 1 have been recognized as carbonyl and phenyl teams, respectively. In contrast with SF supplies, modified SF merchandise exhibited modifications within the amide I, II, and III vibration modes at 1647, 1518, and 1235 cm− 1, respectively. The deconvolution of the height becoming outcomes indicated a rise within the proportion of random coil and α-helix buildings inside TPSF, facilitating the self-assembly into well-defined and morphologically uniform nanoparticles (Fig. S1C-F). Moreover, the electron paramagnetic resonance (EPR) spectrum revealed that the attribute triplet sign attributed to Tpl emerged in TSF and TPSF (Fig. 1E). These findings collectively point out that Tpl and PBAP models have been efficiently modified on SF.
Design, preparation, and characterization of functionalized SF materials (TPSF). (A) Schematics exhibiting the preparation strategy of TPSF. (B) The artificial route for the functionalization of SF to kind TPSF. (C-E) 1H NMR (C), FT-IR (D) and EPR (E) spectra of various supplies together with SF, TSF and TPSF
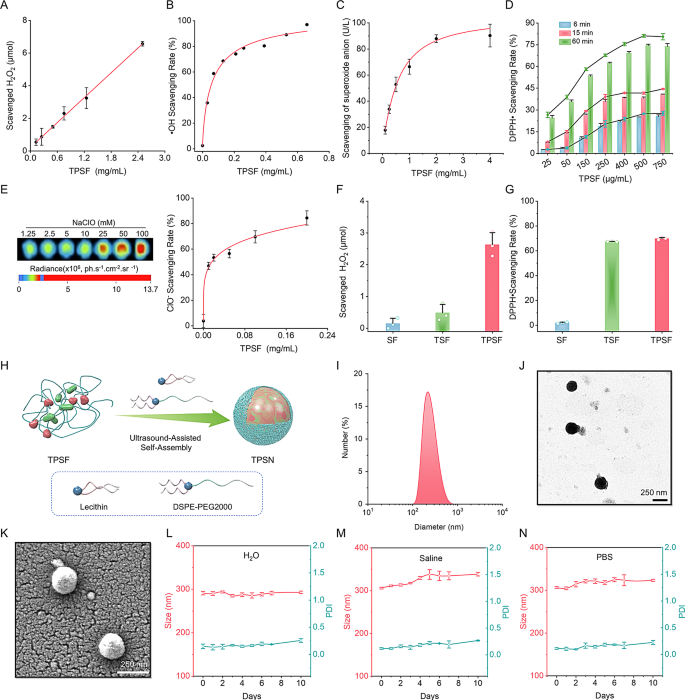
Subsequent, we evaluated the ROS-scavenging capability of TPSF in vitro. Initially, we investigated the H2O2 elimination functionality by incubating TPSF with H2O2 answer for twenty-four h. Upon detecting the residual H2O2 focus, a dose-dependent elimination sample was noticed (Fig. 2A). Moreover, the superoxide anion (O2•−) scavenging capability was assessed. Our outcomes indicated that TPSF was capable of scavenge O2•− in a dose-response method after reacting with the hybrid system for 40 min (Fig. 2C). Moreover, to quantify the free radical and hypochlorite (ClO−) eliminating capabilities of TPSF, established strategies derived from printed literature have been employed [65, 66]. Briefly, OH radical (•OH) was produced by the Fenton response, and methylene blue (MB) served because the •OH indicator probe. When •OH was generated, the colour of combination answer containing MB quickly modified from darkish blue to pale blue. Notably, the colour of combined system confirmed minimal modifications after the addition of 1 mg/mL TPSF and the •OH scavenging charge correlated with the TPSF focus (Fig. 2B; Fig. S2C). Furthermore, 2,2-diphenyl-1-picrylhydrazyl (DPPH•), a nitrogen-free radical, was additionally eradicated by TPSF in a dose and time-associated sample (Fig. 2D). The colour of DPPH• answer shifted from modena to lavender upon the addition of TPSF (Fig. S2A, D). Moreover, a luminescent nanoprobe was employed to detect the capability of TPSF to scavenge hypochlorite (ClO−). As anticipated, TPSF was able to elimination ClO− (Fig. 2E; Fig. S2B). In contrast with SF and TSF alone, TPSF can effectively scavenge each H2O2 and free radical (Fig. 2F, G). Moreover, the outcomes of 1H NMR and HPLC analyses demonstrated that TPSF hydrolyzed to provide HMP (4-hydroxymethylphenol) in a ten mmol/L H2O2 answer (Fig. S3). Collectively, all these outcomes reveal that TPSF possesses a sturdy ROS-scavenging skills.
Evaluation of the ROS-scavenging functionality of TPSF and characterization of TPSN. (A-C) Elimination of H2O2 (A), OH radical (B) and superoxide anion (C) by TPSF after incubation for twenty-four h, 90 min and 40 min, respectively. (D) Focus-dependent and time-dependent scavenging of the DPPH radical by TPSF. (E) Hypochlorite-scavenging effectivity of TPSF at totally different doses after 15 min incubation. The left panel exhibits typical fluorescent photographs at totally different doses of sodium hypochlorite, whereas the proper panel presents a quantitative evaluation of the hypochlorite-scavenging effectivity of TPSF. (F-G) Comparative evaluation of scavenging capabilities of various supplies for H2O2 and DPPH radical. (H) Schematic diagram illustrating the preparation of TPSN. (I-Ok) Measurement distribution profiles (I), consultant TEM (J) and SEM (Ok) picture of TPSN. The imply dimension of TPSN was 291 ± 4 nm and the imply zeta potential was − 33 ± 2 mV. (L-N) The imply dimension distribution of TPSN in deionized water (L), saline (M), PBS (N) throughout 10 days
Preparation and characterization of TPSN
TPSN was fabricated utilizing a modified self-assembly technique, as illustrated in Fig. 2H. Briefly, a small quantity of DSPE-PEG and lecithin answer shaped the aqueous section, into which the TPSF answer was incrementally added. After ultrasound-assisted self-assembly, spherical-like TPSN was obtained, as offered in transmission electron microscopy (TEM) and scanning electron microscopy (SEM) photographs (Fig. 2J, Ok). The crystalline construction of TPSN was evaluated utilizing XRD, which indicated a considerable presence of amorphous areas (Fig. S6C). This low crystallinity of TPSN could improve the susceptibility to degradation in vivo. Dynamic gentle scattering (DLS) evaluation revealed a slim dimension distribution profile with a imply diameter of 291 ± 4 nm (Fig. 2I). Additionally, the zeta potential worth was − 33 ± 2 mV. Notably, the typical dimension of TPSN could possibly be altered by adjusting the TPSF mass and the oil-to-water quantity ratio. For instance, when TPSF mass was 40 mg and 30 mg at an oil-to-water quantity ratio of 1:10, the diameters of the negatively charged TPSN have been 324 ± 0.3 nm and 325 ± 8 nm, respectively, with corresponding polydispersity index (PDI) values of 0.19 ± 0.01 and 0.16 ± 0.04 (Fig. S4A, B, E, F). Whereas the quantity ratio was modified to three:20, the imply sizes have been 291 ± 4 nm and 334 ± 5 nm, with respective PDI values of 0.15 ± 0.04 and 0.17 ± 0.03 (Fig. S4C, D, E, F). General, for the next experiments, TPSN was synthesized utilizing 40 mg of TPSF at a quantity ratio of three:20.
Subsequently, the soundness of TPSN was assessed by monitoring the dimensions and zeta potential modifications of TPSN in distilled water, saline, and PBS at predetermined time factors (Fig. 2L-N, Fig. S6A). The dimensions of TPSN exhibited minimal modifications over a 10-day interval in saline and PBS, whereas it carried out hardly any modifications in distilled water. Moreover, the zeta potential and PDI values remained negatively charged and beneath 0.3, respectively. Moreover, TPSN exhibited stability below weakly acidic and primary situations, and inside a temperature vary from 4 °C to 37 °C at pH = 7.4 (Fig. S6E, F).
Moreover, the ROS-scavenging functionality and hydrolysis profiles of TPSN was additionally examined. Usually, the DPPH• elimination capability of TPSN was much like that of the TPSF materials and nonetheless correlated with the focus of TPSN (Fig. S6B). Subsequently, it was discovered that the hydrolysis charge and diploma of TPSN elevated considerably when incubated with H2O2 at 1 × 10− 2 mol/L. In distinction, when incubated in PBS, a a lot decrease charge of hydrolysis was noticed (Fig. S6D), confirming that TPSN could possibly be ROS responsive hydrolysis.
In vitro anti-oxidative stress and anti-inflammation exercise of TPSN
The pivotal function of inflammatory cells is intracellular ROS elevation [67]. Restoration of intracellular redox homeostasis is a vital technique for attenuating inflammation-associated illnesses. Macrophages and neutrophils play vital roles within the pathogenesis of quite a few inflammatory illnesses. Subsequently, we first examined the mobile uptake of TPSN in macrophages and neutrophils. To quantify and visualize the in vitro phagoptosis of TPSN, comparable Cy5-TPSN was obtained by labeled Cy5 fluorescent dye (Fig. S5A). After incubating Cy5-TPSN with RAW264.7 macrophage cells, mobile internalization was detected by circulation cytometry and confocal laser scanning microscopy (CLSM). It was proven that the internalization of Cy5-TPSN exhibited a time- and dose-dependent sample in RAW264.7 and first intraperitoneal neutrophil cells (Fig. S8, 9). Notably, vital crimson fluorescence was noticed in RAW264.7 cells after incubation with 25 µg/mL of Cy5-TPSN for 1 h. The depth of crimson fluorescence sign elevated with the incubation time and dose of Cy5-TPSN. Based mostly on these findings, the fluorescence depth of Cy5-TPSN was additional quantified by circulation cytometry. Likewise, an identical phenomenon of internalization in major intraperitoneal neutrophil cells was found (Fig. S9). These findings collectively reveal that TPSN may be quickly and successfully phagocytosed by macrophages and neutrophils.
As a result of vital position of ROS in inflammation-related illnesses, we proceeded to evaluate the antioxidant stress capability of TPSN in vitro. 2,7-Dichlorodihydrofluorescein diacetate (DCFH-DA) probe, a fluorescent indicator that indicators the presence of ROS upon oxidation, was used to judge the mobile ROS ranges. In comparison with mannequin group, RAW264.7 cells preincubated with TPSN exhibited considerably decrease fluorescence depth after PMA stimulation (Fig. S12A, B). It was demonstrated that TPSN can successfully scale back the intracellular ROS stage in inflammatory cells. To additional confirm the anti-ROS functionality of TPSN, NAC and nanoparticles ready with totally different modifying supplies have been chosen as concurrent management remedies. NAC was included as a result of its beforehand established antioxidant properties, that are attributed to its capability to raise intracellular GSH ranges [68]. The nanoparticles SN and TSN have been fabricated based mostly on SF and TSF respectively, after which characterised by DLS and TEM (Fig. S5B, C). The imply sizes of SN and TSN have been 223 ± 2.6 nm and 289 ± 2.6 nm, respectively. Concurrently, the zeta-potential values have been evidently adverse, measured at -19.2 mV and − 14.37 mV. As anticipated, TPSN exhibited a superior capability to inhibit the era of intracellular ROS in comparison with each SN and TSN. Moreover, the efficacy of TPSN was corresponding to, or barely exceeded, that of the NAC remedy. (Fig. S12C, D). According to the findings based mostly on circulation cytometric evaluation, fluorescent photographs confirmed weaker fluorescence in cells handled with TPSN (Fig. S12 F, higher row).
Subsequently, we investigated whether or not TPSN might defend macrophages from ROS-induced cell injury. As proven by CCK8 assay, exposing RAW264.7 cells to 2 × 10− 4 mol/L H2O2 and totally different concentrations of TPSN concurrently resulted in a major mobile protecting impact (Fig. S12E). Notably, cells handled with excessive focus of TPSN (50 µg/mL) revealed significantly larger viability in comparison with these handled with recent medium alone. As well as, as demonstrated by circulation cytometry evaluation, incubation with 2 × 10− 4 mol/L H2O2 led to marked apoptosis, notably when in comparison with cells handled with tradition medium alone. Against this, preincubation with TPSN (50 µg/mL) considerably mitigated H2O2-induced cell apoptosis (Fig. S12G, F, decrease row). In the meantime, cells in different teams, together with these handled with TSN and NAC, additionally exhibited decrease apoptosis charges however nonetheless retained the next diploma than these handled with TPSN. Collectively, TPSN revealed apparent cell protecting results by inhibiting mobile ROS era and oxidative stress-mediated cell apoptosis. These findings correspond to the characterization outcomes of TPSF, additional demonstrating its capability to scavenge ROS.
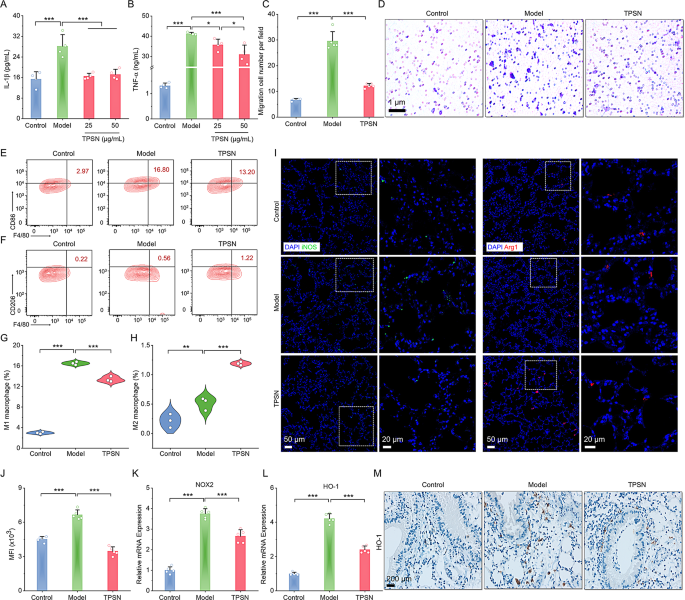
Based mostly on the above outcomes, we subsequently investigated the anti-inflammatory capability of TPSN in vitro. As demonstrated in Fig. 3A-B, the secretion of inflammatory cytokines (IL-1β and TNF-α) elevated dramatically in LPS-stimulated RAW264.7 cells. Conversely, remedy with TPSN resulted in a discount of inflammatory components. In a separate assay, we additionally discovered that the relative mRNA expression ranges of IL-6, IFN-γ, and TGF-β have been decreased in cells handled with TPSN (Fig. S11A-C). As effectively know, macrophage migration performs a vital position in varied inflammatory illnesses as a result of accumulation of pro-inflammatory cytokines [69]. Subsequently, we evaluated the anti-migratory impact of TPSN on cells. Outcomes of transwell assay confirmed that TPSN can inhibit macrophage migration (Fig. 3C, D). Nonetheless, cells within the mannequin group, induced by neutrophils alone, confirmed numerous migrated cells. On the identical time, TPSN can inhibit the migration of neutrophils themselves (Fig. S11D, E). Taken collectively, it signifies that TPSN possesses vital anti-inflammatory and anti-oxidative stress skills.
Evaluation of anti-inflammatory and anti-oxidative stress capabilities and modulation of macrophage phenotype by TPSN remedy. (A-B) The expression ranges of IL-1β (A) and TNF-α within the RAW264.7 cell tradition medium following remedy with varied doses of TPSN. (C) Quantitative evaluation of migrated cells throughout 5 fields. (D) Typical optical microscopy photographs of major neutrophils-induced migration in RAW264.7 cells, positively stained with crystal violet. (E, F) Consultant circulation cytometric profiles illustrating the proportion of M1 (F4/80+CD86+, E) and M2 (F4/80+CD206+, F) macrophages after TPSN remedy. RAW264.7 cells have been stimulated with 100 ng/mL LPS and 5 ng/mL IFN-γ for 33 h, adopted by remedy with 50 µg/mL TPSN for 8 h. (G, H) Quantitative evaluation of M1 and M2 phenotype macrophage, respectively. (I) Immunofluorescence staining of iNOS and Arg1 in lung sections of ALI mice handled with TPSN. The suitable panel signifies zoomed-in photographs of the boxed fields areas akin to fluorescence indicators. Blue, DAPI-nuclear staining; inexperienced, iNOS; crimson, Arg1. (J) The intracellular ROS stage in lung tissues of ALI mice handled with TPSN. Single-cell suspensions of lung tissues have been stained with DCFH-DA and analyzed by circulation cytometry. (Ok, L) The mRNA expression ranges of pro-oxidant genes (NOX2) and antioxidant genes (HO-1) in lung tissues. (M) Immunohistochemical staining of HO-1 in lung sections. Information are offered as imply ± SD (n = 3–5). *p < 0.05, **p < 0.01, ***p < 0.001
Promotion of macrophage phenotype transformation and regulation of redox homeostasis by TPSN
Given the numerous position of macrophages within the development of irritation, we additional investigated the potential of TPSN to modulate macrophage phenotypes. Initially, we employed LPS and IFN-γ as stimulating components to induce the transformation of RAW264.7 macrophages into the M1 phenotype. After co-incubation with 50 µg/mL TPSN, cells have been collected for staining and circulation cytometry evaluation. The outcomes offered in Fig. 3E-H indicated that cells within the TPSN group exhibited a decrease M1 phenotype (F4/80+CD86+) and the next M2 phenotype (F4/80+CD206+) in comparison with the cells stimulated by LPS and IFN-Fγ alone. These outcomes recommend that TPSN can regulate the transformation of pro-inflammatory M1-type macrophages into anti-inflammatory M2-type macrophages in vitro. Moreover, we performed verification of the ends in mice with acute lung harm (ALI). The mice mannequin was established in line with a beforehand described technique, briefly induced with LPS through intranasal supply [65]. Upon comparability of ALI mice handled with TPSN, evaluation of the fluorescence picture demonstrated that ALI mice confirmed stronger iNOS protein (inexperienced fluorescence) and weaker Arg-1 protein indicators (crimson fluorescence sign), which respectively represented the M1 and M2 kind macrophages (Fig. 3I). Completely, TPSN can successfully induce the macrophage phenotype transformation from M1 to M2 types each in vitro and in vivo. Moreover, after incubating a single-cell suspension of lung tissue with DCFH-DA probe, circulation cytometry outcomes confirmed a diminished ROS stage in lung tissue cells from TPSN-treated ALI mice (Fig. 3J). In the meantime, the RT-qPCR analyses demonstrated the downregulation of pro-oxidant genes (NOX2, NADPH oxidase 2) and antioxidant genes (HO-1, heme oxygenase-1) after TPSN remedy (Fig. 3Ok, L). This was additionally in keeping with the immunohistochemistry outcomes of HO-1 (Fig. 3M). These outcomes illustrated that TPSN was capable of inhibit oxidative stress and restore the stability between prooxidants and antioxidants within the lesions. Consequently, TPSN cannot solely facilitate the shift of macrophages towards an anti-inflammatory state but additionally contribute to the restoration of redox stability in injured websites.
Focused remedy of ALI in mice with TPSN
Constructing upon the aforementioned promising outcomes, in vivo research have been carried out to research the therapeutic efficacy of TPSN in experimental animal fashions of inflammatory illnesses. ALI represents a standard life-threatening pulmonary situation characterised by oxidative stress and extreme irritation [70]. Previous to therapeutic analysis, we examined the biodistribution of TPSN initially. After i.v. administration of Cy5-TPSN in regular BALB/c mice, we collected blood samples and main organs for fluorescence imaging. As proven in Fig. S13A, a correspondingly speedy clearance sample from blood was noticed. The fluorescence depth decreased by half after roughly 1.5 h post-injection. Against this, the fluorescent indicators quickly elevated within the main organs, together with lungs and kidneys (Fig. S14).
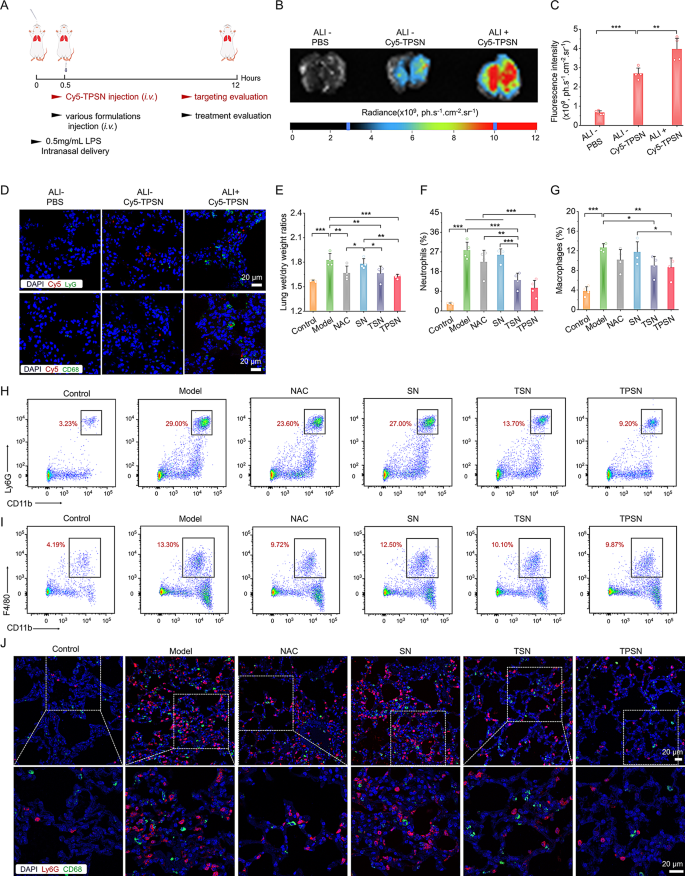
Subsequently, we investigated whether or not TPSN may be successfully gathered at injured lung tissues. All mice have been handled as illustrated in Fig. 4A. We in contrast the fluorescence depth of Cy5 within the lungs of regular and ALI mice administrated with Cy5-TPSN. Outcomes from ex vivo imaging exhibited the buildup of Cy5-TPSN in regular lungs, accompanied by considerably larger fluorescence indicators in injured lung tissues (Fig. 4B, C). It may be attributed to the elevated permeability brought on by the disruption of vascular endothelial and epithelial boundaries in injured lungs. Moreover, immunofluorescence staining was used to judge the mobile distribution of Cy5-TPSN. CD68 and Ly6G have been used to mark the macrophages and neutrophils, respectively. In ALI mice handled with Cy5-TPSN, Cy5 fluorescence was noticed in each neutrophils and macrophages (Fig. 4D). In distinction, no fluorescence indicators have been present in each cells of regular mice. Moreover, a relatively larger stage of Cy5 fluorescence was noticed in lung cryosections of ALI mice. Furthermore, TPSN was confirmed to be engulfed by neutrophils and macrophages in cell experiments. Collectively, these outcomes indicated that phagocytosis of inflammatory cells can improve the injured web site concentrating on capability of TPSN. Moreover, mobile uptake of Cy5-TPSN by pulmonary microvascular endothelial cells (PMVEC) occurred in a dose- and time-dependent method (Fig. S10). Subsequently, TPSN can successfully gathered in broken lungs, thereby offering a basis for subsequent therapeutic research.
In vivo concentrating on efficiency and therapeutic efficacy of TPSN in ALI mice after i.v. administration. (A) Schematic illustration of the institution of ALI mice mannequin and remedy regimens. (B, C) Typical ex vivo photographs (B) and quantitative evaluation (C) indicating the buildup of Cy5-TPSN within the lungs at 12 h after i.v. administration of Cy5-TPSN. (D) Confocal microscopy photographs of lung cryosections exhibiting the co-localization evaluation between Cy5-TPSN and CD68+ macrophages or Ly6G+ neutrophils. (E) The lung wet-to-dry weight ratios after totally different remedy. (F-I) Stream cytometric quantitative evaluation and typical dot plots of neutrophil (F, H) and macrophage (G, I) proportions in pulmonary tissues of ALI mice. (J) Confocal microscopy photographs of CD68+ macrophages and Ly6G+ neutrophils in lung cryosections of ALI mice. The ALI mice mannequin was established with 50 µL LPS at 0.5 mg/mL through intranasal supply. After 0.5 h, mice obtained totally different formulations by i.v. injection. Within the therapeutic efficacy assay, mice within the mannequin group obtained saline, whereas the opposite teams have been handled with NAC, SN, TSN, or TPSN at a dosage of two mg/kg, respectively. At 12 h after varied remedies, mice lungs have been collected for quantitative and histological analyses. Information are offered as imply ± SD (n = 4). *p < 0.05, **p < 0.01, ***p < 0.001
Inspired by the environment friendly anti-inflammatory and concentrating on functionality of TPSN, the therapeutic impact of TPSN was investigated in ALI mice. The degrees of pro-inflammatory cytokines IL-1β, TNF-α, and MCP-1 in bronchoalveolar lavage fluid have been clearly decreased after remedy with TPSN at a dosage of two mg/kg (Fig. S15). In distinction, administration of TPSN at 0.5 mg/kg didn’t yield a therapeutic final result, apart from a discount in IL-1β ranges.
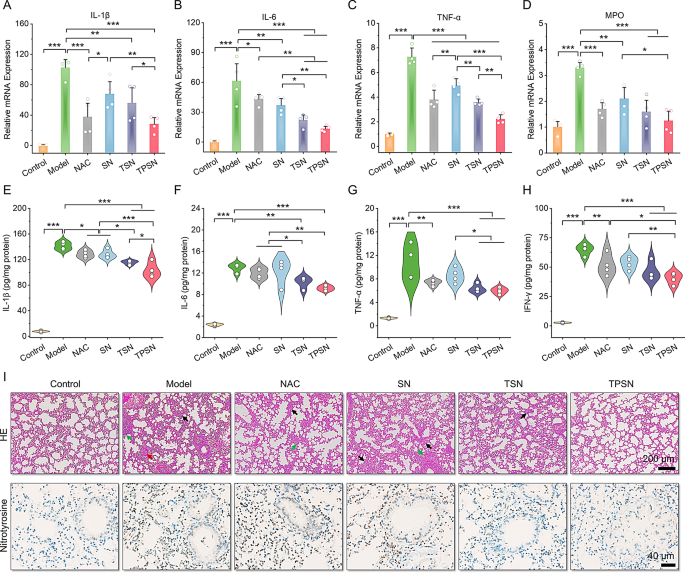
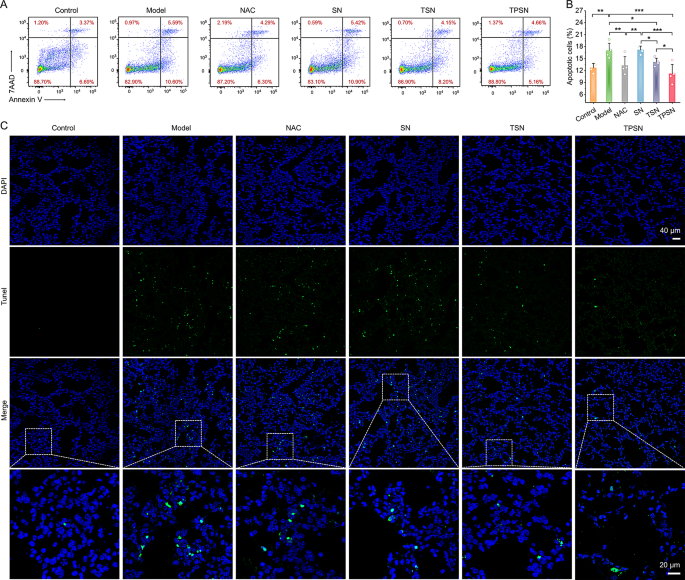
Moreover, the therapeutic efficacy of TPSN was additionally in contrast in additional element with these of NAC, SN, and TSN. The lung moist/dry weight ratio, an index of pulmonary edema brought on by acute irritation, was remarkably decreased by remedy with 2 mg/kg TPSN and NAC, in comparison with ALI mice handled with saline (Fig. 4E). Equally, TSN remedy might additionally enhance the pulmonary edema of ALI mice, apart from SN remedy. These outcomes have been in keeping with the narrow-spectrum ROS-scavenging capability of TSN. As talked about above, ALI is mechanistically related to pulmonary infiltration of immune cells, comparable to neutrophils and macrophages [70]. Extreme infiltration normally causes injury to lung tissues due to the massive manufacturing of pro-inflammatory cytokines and chemokines [71]. Thus, the neutrophils and macrophages in lung tissues have been measured by circulation cytometry evaluation and immunofluorescence staining. In comparison with regular mice, a major improve in neutrophils and macrophages was noticed in ALI mice handled with saline (Fig. 4F-I). Nonetheless, TPSN remedy successfully decreased the proportion of neutrophils and macrophages to 10.45 ± 3.86% and eight.64 ± 2.12%, respectively. Likewise, TSN, with restricted ROS-scavenging functionality, displayed comparatively weaker inhibition of inflammatory cell infiltration. Of notice, each of them have been superior to NAC remedy. Nonetheless, remedy with SN didn’t reveal any therapeutic impact. According to the circulation cytometric evaluation outcomes, the therapeutic impact of TPSN was additionally confirmed in immunofluorescence staining (Fig. 4J). Remedy with TPSN resulted within the weaker expression of CD68 (inexperienced fluorescence) and Ly6G (crimson fluorescence) within the sections of lung tissues. These outcomes have been in accordance with the decreased ranges of inflammatory cytokine (Fig. 5A-H; Fig. S16). The gene expression of pro-inflammatory components, together with IL-1β, IL-6, TNF-α, and MPO, was considerably downregulated after TPSN remedy. In the meantime, comparable outcomes have been discovered by way of measuring the extent of inflammatory components in serum and lung tissue homogenates by ELISA. Additional hematoxylin and eosin (H&E)-stained sections have been used to judge the pathological modifications in lung tissues. As illustrated in Fig. 5I (higher panel), the lung tissues of ALI mice exhibited notable pathological modifications, together with vital infiltration of neutrophils (indicated by inexperienced arrow), thickened alveolar partitions, collapsed alveolar, and parenchymal lesions (indicated by black arrow), together with numerous necrotic epithelial cells and infiltrated neutrophils filling the bronchial lumen (indicated by crimson arrow). Whereas just a few histological abnormalities have been noticed after TPSN remedy. Moreover, immunohistochemical staining of nitrotyrosine was carried out to judge the anti-oxidative stress functionality of TPSN (Fig. 5I, decrease panel). Nitrotyrosine, thought of a delicate biomarker of oxidative stress, is shaped by peroxynitrite-mediated nitration on protein tyrosine residues. In comparison with NAC, SN, and TSN remedies, TPSN remedy resulted in a decrease expression of nitrotyrosine, nearly equal to that of regular mice. Furthermore, therapeutic results of TPSN have been additionally confirmed by detecting apoptotic cells in lung tissues. Notably, circulation evaluation revealed that TPSN remedy mitigates cell apoptosis within the lung tissue of ALI mice (Fig. 6A, B). TdT mediated dUTP nick-end labeling (TUNEL) staining of the lung tissue sections exhibited comparable outcomes (Fig. 6C). In comparison with regular mice or TPSN-treated ALI mice, a lot stronger TUNEL optimistic indicators have been present in ALI mice. Likewise, SN confirmed no anti-apoptotic results, and TSN might partly defend cells from apoptosis. Collectively, TPSN demonstrated superior tissue safety capabilities in comparison with NAC and TSN remedy, as evidenced by decrease lung moist/dry weight ratios, diminished pulmonary infiltration of inflammatory cells, decreased secretion of pro-inflammatory components, inhibited formation of oxidation merchandise, and mitigated cell apoptosis in lung tissues. Consequently, these outcomes reveal that TPSN, with successfully lung concentrating on capability and anti inflammatory properties, holds promise as a possible therapeutic technique for ALI.
Analysis of the anti-inflammatory impact of TPSN and histological evaluation in ALI mice. (A-D) Quantification of mRNA ranges for IL-1β (A), IL-6 (B), TNF-α (C) and MPO (D) in lung tissues of ALI mice following totally different remedies. (E-H) Serum expression ranges of IL-1β (E), IL-6 (F), TNF-α (G) and IFN-γ (H) in ALI mice as measured by ELISA. (I) H&E staining (higher panel) and nitrotyrosine immunohistochemically staining (decrease panel) of lung tissue sections. Black arrows point out alveolar wall thickening and substantial lung tissues. Inexperienced arrows point out areas of neutrophils infiltration. Purple arrows level to accumulative necrotic epithelial cells and extravasated neutrophils within the bronchial lumen. Information in (A-H) are imply ± SD (n = 4). *p < 0.05, **p < 0.01, ***p < 0.001
Evaluation of anti-apoptotic functionality of TPSN in lung tissues from ALI mice subjected to numerous formulations. (A-B) Stream cytometric profiles (A) and quantitative information (B) of apoptotic lung tissue cells after totally different remedies. (C) TUNEL-stained evaluation of lung sections. Information are imply ± SD (n = 4). *p < 0.05, **p < 0.01, ***p < 0.001
Therapeutic effectivity of TPSN in Cisplatin-Induced AKI mice
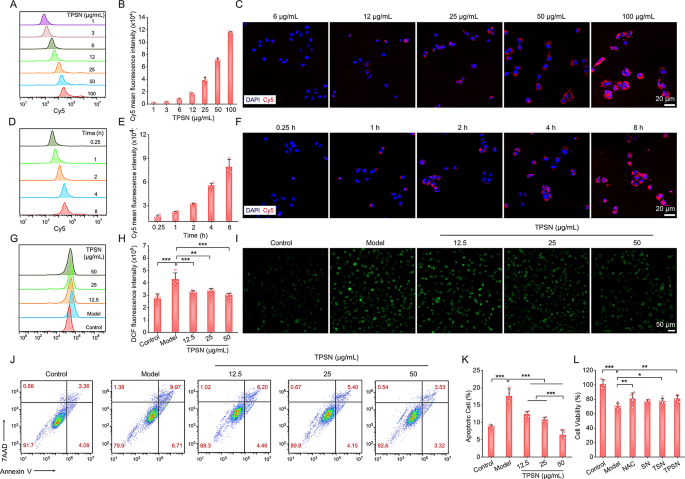
To validate the universality of the aforementioned silk fibroin-based nanomedicine, we examined the therapeutic efficacy of TPSN in a murine mannequin of cisplatin-induced acute kidney harm (AKI). It’s well-documented that oxidative stress and irritation are vital components within the pathogenesis of cisplatin nephrotoxicity [72]. This nephrotoxicity represents a major antagonistic impact on regular organs in sufferers present process cisplatin-based chemotherapy, considerably impacting their scientific outcomes [72]. Subsequently, the cytoprotective impact of TPSN was evaluated in cisplatin-induced cell injury. Contemplating that the renal epithelial cells are prone to oxidative stress in AKI, we used HK-2 cells to review cytoprotective properties of TPSN towards ROS injury. As proven in Fig. 7A-F, HK-2 cells can endocytose TPSN in a dose- and time-dependent method, as evidenced by circulation cytometry and confocal microscopy evaluation. Subsequent, we examined the inhibitory impact of TPSN on intracellular ROS manufacturing (Fig. 7G-I). After remedy with 10 µmol/L cisplatin, HK-2 cells confirmed a dramatically elevated in intracellular ROS. Compared, when cells have been co-incubated with TPSN, the intracellular ROS considerably decreased. Qualitative evaluation of intracellular ROS was confirmed by fluorescence imaging. According to our expectations, the ROS indicators (inexperienced fluorescent) have been markedly diminished following TPSN remedy. Furthermore, circulation cytometry evaluation was employed to evaluate the inhibition functionality of TPSN towards cisplatin-induced cell apoptosis and necrosis (Fig. 7J, Ok). It clearly demonstrated that cisplatin can promote HK-2 cells apoptosis and necrosis. Against this, the apoptotic ratio of HK-2 cells was considerably decreased after remedy with TPSN. Notably, the high-dose TPSN (50 µg/mL) confirmed a greater cytoprotective impact than different doses. In the meantime, we moreover in contrast cytoprotective impact of TPSN with NAC, SN, and TSN in CCK8 assay. As illustrated in Fig. 7L, cisplatin markedly diminished the cell viability of HK-2 cells, and this decline was noticeably alleviated by TPSN. For different remedies, NAC and TSN exhibited a cytoprotective impact, in keeping with their inhibition of H2O2-induced apoptosis in RAW264.7 cells. Taken collectively, these outcomes reveal that TPSN can defend cells towards cisplatin-induced cell injury by decreasing intracellular ROS ranges, inhibiting apoptosis, and stopping necrosis.
Analysis of cytoprotective results of TPSN in cisplatin-induced HK-2 cells injury. (A, B) Stream cytometric profiles (A) and quantification counts (B) indicating dose-dependent mobile uptake of Cy5-TPSN in HK-2 cells after 2 h incubation. (C) Fluorescence photographs exhibiting dose-dependent internalization of Cy5-TPSN in HK-2 cells. (D, E) Typical circulation cytometric curves (D) and quantitative evaluation (E) of time-dependent mobile uptake of Cy5-TPSN at 25 µg/mL. (F) Confocal microscopy photographs indicating time-dependent internalization of Cy5-TPSN at 25 µg/mL in HK-2 cells. (G, H) Stream cytometry profiles (G) and quantitative information (H) of intracellular ROS era in HK-2 cells after remedy with totally different dose of TPSN. (I) Fluorescence photographs exhibiting a consultant discount in intracellular ROS era after TPSN remedy. The HK-2 cells have been stimulated by 10 µmol/L cisplatin, adopted by co-incubation with TPSN for twenty-four h. (J, Ok) Typical circulation cytometric dot plots (J) and quantitative evaluation (Ok) of apoptotic HK-2 cells after TPSN remedy. The HK-2 cells have been induced with 15 µmol/L cisplatin, after which co-incubated with TPSN for twenty-four h. (L) Cell viability of HK-2 cells uncovered to fifteen µmol/L cisplatin and totally different formulations for twenty-four h. Information are imply ± SD (n = 3–4). *p < 0.05, **p < 0.01, ***p < 0.001
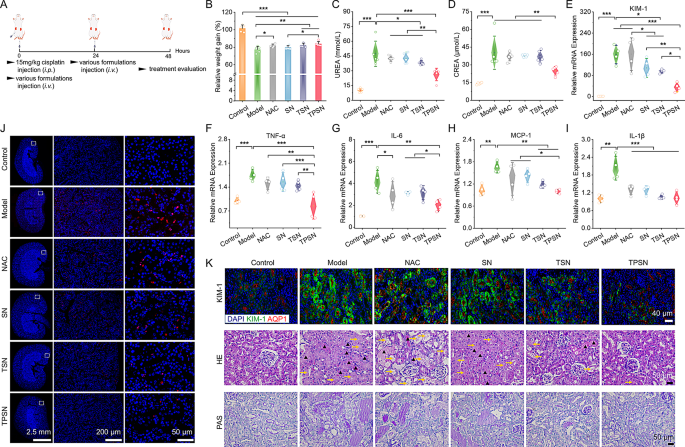
In keeping with a reported established technique, AKI was induced in mice by intraperitoneal injection of a single excessive dose of cisplatin (15 mg/kg, Fig. 8A). Based mostly on the outcomes of pharmacokinetics and biodistribution of TPSN, we noticed a stronger accumulation of Cy5-TPSN in injured kidneys in comparison with wholesome kidneys (Fig. S13B, C). These outcomes have been confirmed by immunofluorescence evaluation (Fig. S13D). All of those findings recommended that TPSN may be effectively focused to injured kidneys. Subsequent, we assessed the therapeutic impact of TPSN in AKI mice. After remedy with TPSN at 2 mg/kg, the degrees of serum urea (UREA), serum creatinine (CREA), and IL-1β have been considerably decreased (Fig. S17). Properly-documented scientific indicators of renal dysfunction embrace excessive ranges of UREA and CREA [73]. Against this, TPSN at a dose of 0.5 mg/kg solely confirmed a discount in CREA and IL-1β expression.
Therapeutic results of i.v. delivered TPSN in AKI mice. (A) Schematic illustration of the institution and remedy regimens for cisplatin-induced AKI in mice. (B) Relative modifications in physique weight of AKI mice after totally different remedies. (C, D) Serum expression ranges of UREA (C) and CREA (D) in AKI mice after varied remedies. (E-I) Relative mRNA expression ranges of KIM-1 (E), TNF-α (F), IL-6(G), MCP-1 (H) and IL-1β (I) in kidneys of AKI mice handled with distinct formulations. (J) TUNEL staining of the kidney cryosections from AKI mice. (Ok)Staining photographs of kidney sections marked with KIM-1 antibody (higher panel), H&E (center panel) and PAS (decrease panel). Aquaporin-1 (AQP1) is a biomarker of proximal tubule cells. The yellow arrows represented tubules with necrosis, epithelial anoikis cavitation or lack of brush border. Triangles indicated casts formation in tubes. Mice in mannequin group have been obtained with saline, whereas different teams have been respectively handled with NAC, SN, TSN or TPSN at 2 mg/kg. Information are imply ± SD (n = 4–5). *p < 0.05, **p < 0.01, ***p < 0.001
Moreover, in a separate experiment, we in contrast the therapeutic results of TPSN with NAC, SN, and TSN at a dose of two mg/kg. As anticipated, AKI mice handled with TPSN or TSN confirmed aid from weight reduction, whereas AKI mice (saline handled) exhibited dramatic body weight loss inside 48 h (Fig. 8B). As indicated in Fig. 8C-D, remedy by TPSN considerably decreased the degrees of UREA and CREA in AKI mice, demonstrating that TPSN might successfully restore renal operate. By comparability, TSN solely partially diminished the serum UREA stage, indicating its restricted capability for ROS-scavenging. Equally, NAC and SN didn’t present any therapeutic impact. Moreover, the gene expression of pro-inflammatory components, together with TNF-α, IL-6, MCP-1, and IL-1β, was markedly inhibited by TPSN (Fig. 8F-I). This outcome was verified by immunohistochemistry staining for IL-6 (Fig. S19). Furthermore, circulation cytometry was used to investigate the infiltration of neutrophils and macrophages. According to the RT-qPCR outcomes, TPSN demonstrated the potential to inhibit neutrophil and macrophage infiltration (Fig. S18A-C). Kidney harm molecule-1 (KIM-1) is a novel biomarker that’s intently related to the renal tissue injury [74]. Certainly, the gene expression of KIM-1 in AKI mice was dramatically elevated in comparison with that in mice handled by TPSN (Fig. 8E). In the meantime, immunofluorescence evaluation confirmed comparable expression patterns of KIM in AKI mice after TPSN remedy (Fig. 8Ok). In addition to, histological analyses have been performed utilizing H&E and PAS staining (Fig. 8Ok). Kidneys of AKI mice represented irregular structure with outstanding necrosis, epithelial anoikis cavitation, or lack of brush border in tubules (proven as yellow arrow). And the formation of solid buildings in tubules (indicated by black triangles) was additionally famous, a function that’s thought to be a major pathological indicator of renal harm. Equally, PAS staining additionally revealed a considerable amount of glycogen deposition within the kidney tubules of AKI mice. Of notice, all these histological abnormalities have been successfully reversed by TPSN remedy.
The position of oxidative stress in cisplatin-induced nephrotoxicity is effectively established. Extreme accumulation of ROS throughout the proximal tubules triggers tubular cell apoptosis [72]. As illustrated by the TUNEL assay, apoptotic cells in TPSN group have been clearly decreased in comparison with AKI mice handled with saline (Fig. 8J; Fig.S18E). In the meantime, the H2O2 stage within the homogenate of renal tissue was additionally measured. As anticipated, the H2O2 stage in AKI mice was considerably diminished after TPSN remedy (Fig. S18D). General, AKI mice handled with different formulations solely confirmed a major lower in among the parameters assessed. Just like the findings in ALI fashions, remedy of cisplatin-challenged mice with TPSN achieved fascinating protecting results, as evidenced by notable reductions within the ranges of UREA, CREA, KIM-1, H2O2, pro-inflammatory cytokines, and kidney tissue harm. Taken collectively, these outcomes reveal that TPSN might successfully restore renal operate in AKI mice.
Biocompatibility of TPSN
In gentle of its fascinating anti-oxidative stress and anti inflammatory actions in vitro and vivo, each animal experiments and in vitro assays have been carried out to evaluate the security of TPSN. Firstly, the cytotoxicity of TPSN was evaluated in RAW264.7 and HK-2 cells. After incubation for six h and 12 h in RAW264.7 cells, comparatively excessive cell viability was noticed no matter totally different doses of TPSN (Fig. S7A, B). Even at a dose of 250 µg/mL, 5-fold relative to the mobile administrated dose, the proportion of viable cells remained larger than 90% after incubation with TPSN for 12 h. Equally, HK-2 cells incubatied with TPSN for 8 h confirmed negligible cytotoxicity (Fig. S7C). Moreover, the hemolysis assay in vitro confirmed no hemolysis even at dose as excessive as 2 mg/mL of TPSN (Fig. S7D).
Furthermore, acute toxicity assessments have been performed in wholesome mice following i.v. injection of TPSN at 100 and 200 mg/kg, respectively. Mice in all teams maintained their typical every day eating regimen and water consumption with out experiencing weight reduction or any basic behavioral issues (Fig. S20A). On day 14 after administration, the mice have been euthanized. The blood routine check evaluation revealed no vital alternations in typical hematological parameters, comparable to platelet (PLT), crimson blood cell (RBC), and hemoglobin (HGB) ranges (Fig. S20B-D). Furthermore, the biochemical parameters related to liver and kidney capabilities additionally confirmed no distinct variations between teams (Fig. S20E-H). In the meantime, examination of H&E-stained sections of the mind, coronary heart, liver, spleen, lungs, and kidneys revealed negligible accidents and pathological patterns (Fig. S21). As well as, monitoring the biochemical parameters associated to liver and kidney operate on days 1, 3, 5, and seven post-administration revealed no vital variations between the teams (Desk S2). Moreover, TPSN can degrade into small molecule HMP within the homogenates of lung, kidney, and liver tissues (Fig. S22). General, these preliminary information point out that TPSN has an excellent security profile after i.v. administration, with no noticed cytotoxicity or in vivo systemic toxicity, even at doses 50–100 occasions larger than the therapeutic dose. This extra proof substantiates the security and efficacy of our proposed technique for establishing bioactive nanoparticles using silk fibroin.