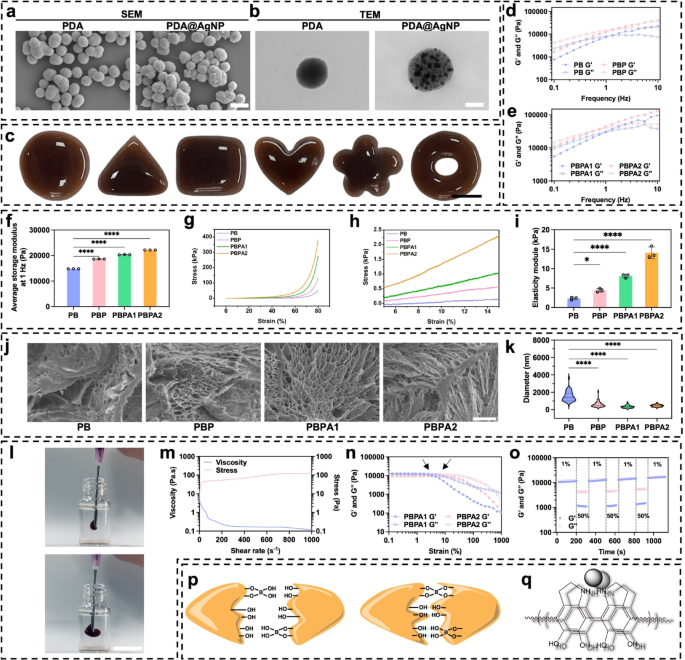
Synthesis and characterization of PDA@AgNP nanozyme
PDA stabilizes AgNPs towards aggregation and oxidation by means of catechol/amine-mediated anchoring, whereas its π-π stacking and hydrogen bonding improve colloidal stability. Redox-active catechol teams additional quench radicals and synergize with AgNP catalysis, boosting oxidative stability and adaptive catalytic efficiency. These properties make PDA-AgNP composites extremely efficient for sensible functions [39]. PDA is self-assembled in an alkaline atmosphere from dopamine, adopted by the deposition of AgNP by means of one-step discount (Fig. S1). Underneath scanning electron microscope (SEM), PDA self-assembly is famous, and the grey represents AgNP indicating profitable synthesis of PDA@AgNP (Fig. 1a and Fig. S2). For clearer visualization, transmission electron microscopy (TEM) confirmed ample AgNP (Fig. 1b and S3). After attaching AgNP, the common particle measurement of PDA@AgNP barely elevated (Fig. S4). Power dispersive spectrometry (EDS) of PDA@AgNP revealed that the entire mass fraction of silver was 17.7% (Fig. S5), and the inclusion resulted in adjustments in zeta potential (Fig. S6) and UV-vis absorption (Fig. S7) showcasing the profitable AgNP deposition. The mixing of PDA and AgNPs enhances PCE by accelerating cost switch [40], according to noticed superior photothermal efficiency of PDA@AgNP over PDA alone in aqueous environments (Fig. S8).
These outcomes point out profitable PDA@AgNP fabrication, and the addition of AgNP considerably enhances PCE, offering basis for environment friendly biofilm disruption. To completely make the most of the potential of PDA@AgNP, incorporating it into hydrogels prevents PDA self-aggregation [41].
Characterization, and mechanical properties of PDA@Ag and PBPA hydrogel. a) SEM of PDA and PDA@AgNP, scale bar = 400 nm; b) TEM of PDA and PDA@AgNP, scale bar = 110 nm; c) Plasticity of PBPA2 hydrogel, scale bar = 1 cm; d) Frequency sweep take a look at (from 0.1 to 10 Hz) at 37 °C of PB and PBP; e) Frequency sweep take a look at (from 0.1 to 10 Hz) at 37 °C of PBPA1 and PBPA2; f) Quantitative common storage modulus at 1 Hz of the hydrogels, n = 3; g) Compression take a look at with stress-strain curve from 0% to 80% of various hydrogel; h) pressure from 5% to fifteen% of compression take a look at of various hydrogel; i) Elasticity modulus of various hydrogels within the 5–15% pressure vary of compression curves, n = 3; j) SEM photographs of various hydrogels, scale bar = 5 μm; okay) The pore measurement of the hydrogel with totally different parts, n = 50. l) injection of PBPA2 hydrogel, scale bar = 1 cm; m) viscosity measurements of PBPA2 with rising shear charge at a set frequency of 1 Hz below continues stress; n) Rheological properties of the hydrogel in pressure amplitude sweep (γ = 0.1–1000%) at 1 Hz; o) Rheological property of PBPA2 at low-1% and high-50% strains; p) Dynamic hydrogen and B-O bond of PBPA; q) π-π stacking of PDA@AgNP. Knowledge represented imply ± SD. **** p < 0.0001, *** p < 0.001, ** p < 0.01, * p < 0.05
Fabrication, characterization, and mechanical properties of PBPA hydrogel
PVA, valued for its biocompatibility and biodegradability [42], was crosslinked through dynamic BEB to introduce ROS responsiveness [43]. Borax-mediated BEB formation between PVA diol teams and PDA@AgNP (Fig. S9) yields a hydrogel steady in impartial to barely alkaline circumstances but degradable below acidic, ROS-rich wound environments [44]. ROS-triggered BEB cleavage allows managed nanozyme launch [45, 46].
Fourier remodel infrared spectroscopy (FTIR) confirmed profitable BEB crosslinking, indicated by the height at 1334 cm−1 (Fig. S10). To probe component-specific roles, hydrogels with various compositions have been ready: PB (PVA-BEB-PVA), PBP (PVA-BEB-PDA), PBPA1 (PVA-BEB-1 unit PDA@ AgNP), and PBPA2 (2 items). Enhanced BEB crosslinking was noticed with PDA presence and rising PDA@AgNP content material, evidenced by elevated intensities at 1334 cm−1 and 1660 cm−1 (C = O stretch) (Fig. S11) [47], which was highest in PBPA2 as a result of elevated quantity of PDA.
Hydrogels containing totally different parts have been efficiently synthesized (Fig. S12) with wonderful plasticity (Fig. 1c), permitting them to be personalised to wound shapes. This distinctive plasticity originates from the distinctive viscoelastic properties exhibited by PVA and BEB (Fig. 1d and e), permitting the fabric to move like a liquid but retain form after yielding. The common G’ at 1 Hz elevated when addition of BEB and PDA@AgNP (Fig. 1f), as a consequence of further crosslinking launched by catechol teams from PDA [45, 46]. PDA enhances power dissipation, rising each G′ and G′′. AgNPs act as bodily crosslinking websites, uniformly dispersed to limit polymer chain mobility and additional increase power dissipation through interfacial interactions [47]. This synergy considerably improves mechanical properties, mirrored within the highest compressive power at 80% deformation for PBPA2 (Fig. 1g), possible as a consequence of strongest crosslinking. Further reinforcement arises from hydrogen bonding and π-π stacking between PDA and PVA [48]. Elasticity modulus will increase considerably which additionally attributed to those causes (Fig. 1h and that i). Critically, extended publicity to excessive modulus (>50 kPa) induce mechanical reminiscence in fibroblasts, selling myofibroblast activation and sustained fibrosis [49]. Particularly, low modulus and degradability keep away from initiating this mechanical stress-myofibroblast activation-collagen synthesis suggestions loop. By stopping persistent myofibroblast exercise, the system helps scar-inhibited regenerative therapeutic, addressing a key problem in wound therapy [49]. Regardless of the strongest crosslinking, the elastic module of PBPA2 is lower than 20 kPa (Fig. 1i). Furthermore, every hydrogel maintained excessive stretchability, extending almost tenfold (Fig. S13 and S14). The sturdy plasticity is probably going due to the freeze-casting, permitting PVA to kind micro-scale honeycomb-like pore partitions consisting of interconnected nanofibril meshes (Fig. 1j and S15) [50]. The pore measurement of the PVA hydrogel decreases with dopamine addition (Fig. 1okay), primarily as a consequence of elevated crosslinking from hydrogen bonding, BEB, and π-π interactions. Introduction of PDA enhanced community compactness, whereas additional incorporation of PDA@AgNP tightened the construction through pseudocrosslinking mediated by nanoparticle–polymer dynamic interactions [51]. Doubling PDA@AgNP focus elevated crosslinking density, selling additional densification. Regardless of lowered pore measurement, injectability was maintained. The hydrogel exhibited shear-thinning conduct below rising shear charges (Fig. 1l and m), enabled by reversible BEB breakage [44, 52]. This was enhanced by PVA viscoelasticity and PDA interactions. Fast post-injection structural restoration was facilitated by hydrogen bonding and π-π stacking by means of PDA, supporting self-healing (Fig. S16). Incorporation of AgNPs additional enhanced self-healing by re-establishing conductive networks upon materials rejoining enabling restoration inside seconds [53]. After full rupture below shear (Fig. 1n), the hydrogel quickly self-healed upon drive removing (Fig. 1o and S17). As talked about above, these mechanical properties have been primarily due to BEB, hydrogen bonds, and π-π stacking (Fig. 1p and q).
Whereas the hydrogel’s skin-compatible modulus facilitates wound utility, its comparatively low mechanical power could compromise stability. Rheological analysis below diversified circumstances confirmed retained viscoelasticity after one month in sealed storage (Fig. S18). Nonetheless, rigidity elevated after at some point in dry air, possible as a consequence of water loss. In moist circumstances simulating wound exudate, each G′ and G′′ decreased markedly, suggesting swelling-induced structural enlargement and partial matrix degradation. After NIR irradiation, hydrogel rigidity elevated considerably. Photothermal conversion by AgNP and PDA elevated native temperature, enhancing chain mobility and selling reorganization of dynamic BEB right into a denser community. Hydrogen bonding and BEB stability additionally improved, additional rising crosslinking density. Elevated temperature may also induce polymer rearrangement or section transition, resulting in community compaction [54, 55]. These mechanisms collectively account for the rise in G′ post-irradiation.
In abstract, PBPA displays outstanding plasticity, tensile power, self-healing properties, and injectability, which fulfill its use in numerous wounds. Its viscoelastic nature ensures conformal pores and skin protection, whereas speedy self-healing withstands repeated mechanical stress at joint websites. The low module, steady throughout numerous circumstances, avoids therapeutic suppression and minimizes native rigidity. These superior mechanical properties give hydrogel sturdy basis for wound utility.
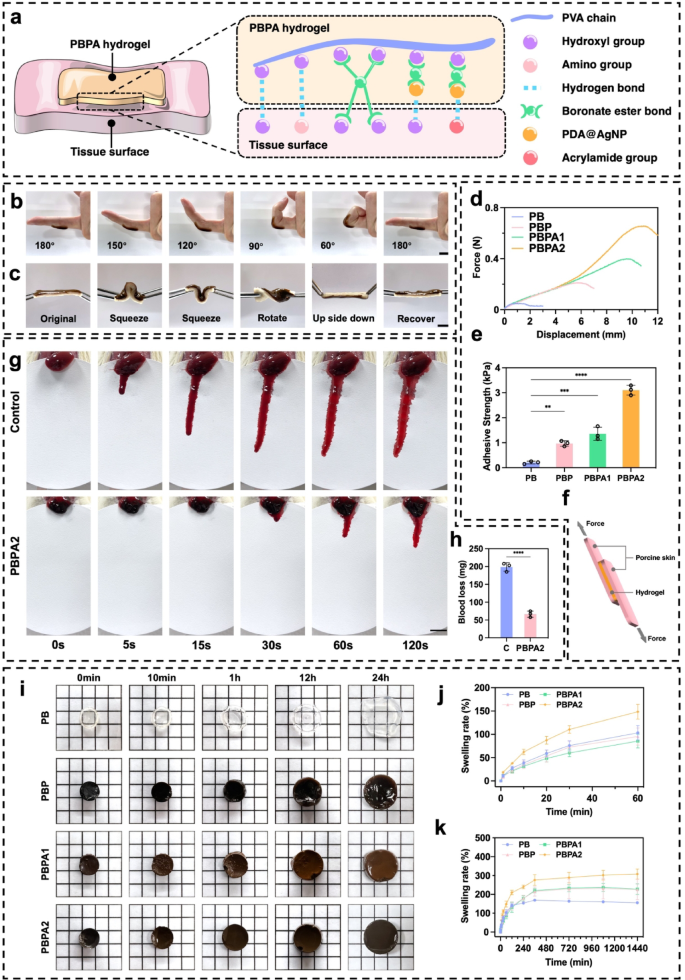
Adhesiveness, hemostasis, and swelling means of PBPA hydrogel
The PBPA hydrogel displays sturdy adhesion by means of a number of mechanisms (Fig. 2a). PDA and PVA present ample hydroxyl and amino teams that kind hydrogen bonds with polar pores and skin residues [56, 57]. Dynamic BEB react reversibly with pores and skin hydroxyl teams, providing strong but adaptive adhesion. Though AgNPs don’t immediately contribute to adhesion, their antibacterial motion helps preserve interfacial cleanliness, supporting sustained adherence. Moreover, the micro-rough floor construction from directional freezing (Fig. 1j), enhancing bodily interlocking. The hydrogel maintains agency adhesion throughout joint flexion and totally recovers after bending at numerous angles (Fig. 2b and c), indicating its suitability for masking pores and skin, particularly on continuously bent joints. To evaluate its adhesiveness, the hydrogel was evenly utilized between two items of porcine pores and skin (Fig. S19 and 2f). Stretching checks revealed a major enhance in adhesiveness upon the addition of PDA, with PBPA2 displaying the most effective efficiency, characterised by the longest stretch distance and highest tensile stress (Fig. 2d), together with elevated adhesive power (Fig. 2e). This distinctive adhesive efficiency was additionally noticed in different organs and supplies (Fig. S20 and S21).
The PBPA hydrogel demonstrated efficient hemostatic capability in a rat liver bleeding mannequin (Fig. 2g), considerably decreasing complete blood loss inside two minutes (Fig. 2h). This impact is probably going attributable to its sturdy tissue adhesion and speedy fluid absorption, which assist take away exudate, inhibit eschar formation, and eradicate microbial niches, thereby supporting subsequent regeneration [1]. The hydrogel maintained water absorption for as much as 24 h (Fig. 2i). PBPA2 confirmed the best swelling ratio each initially (Fig. 2j) and at 24 h (Fig. 2okay), whereas retaining structural integrity (Fig. 2i), possible as a consequence of its greater PDA content material and compact pore construction. Moreover, PBPA2 exhibited superior water retention, preserving ~ 50% absorbed water after one week (Fig. S22), owing to its dense, dual-crosslinked porous community [58]. This moisture-locking functionality prevents desiccation, sustains a moist therapeutic atmosphere, and minimizes syneresis, guaranteeing constant wound contact [59].
The PBPA hydrogel achieves speedy hemostasis and effectively absorbs wound exudate, key drivers of eschar formation. By eliminating these elements, it suppresses eschar growth whereas sustaining a moist, conforming wound interface. This hydrated, eschar-free microenvironment facilitates cell migration and re-epithelialization, offering best circumstances for regeneration and selling therapeutic with lowered scarring.
Adhesiveness, hemostatic, fluid absorption, and swelling means of the hydrogel. a) Attainable adhesive mechanisms of PBPA hydrogel to tissue. b) Software of PBPA2 hydrogel when bending joint, scale bar = 1 cm; c) Software of PBPA2 hydrogel on porcine pores and skin, scale bar = 1 cm; d) Pressure-displacement curves of various hydrogels-bonded porcine pores and skin; e) Quantitative adhesive power of the hydrogels calculated from the force-displacement curves, n = 3; f) Schematic determine of the lap shear adhesive take a look at utilizing porcine pores and skin and the hydrogel; g) Consultant photographs of the hemostatic take a look at utilizing rat liver, scale bar = 1 cm; h) Quantitative blood loss, n = 3; i) Consultant photographs of various hydrogels emerged in PBS at totally different time factors, measurement of small sq. = 5 mm*5 mm; j) Quantitative swelling evaluation of various hydrogels from 0–60 min, n = 3; okay) Quantitative swelling evaluation of various hydrogels from 0–24 h, n = 3; Knowledge represented imply ± SD. **** p < 0.0001, *** p < 0.001, ** p < 0.01, * p < 0.05
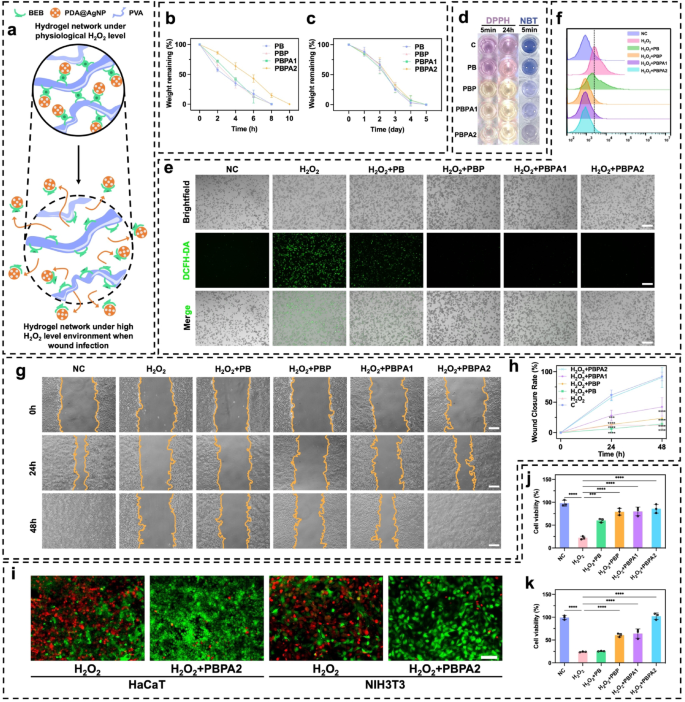
In vitro validation of ROS-responsive nanozyme supply and ROS-scavenger properties of PBPA hydrogel
The PBPA hydrogel dynamically regulates ROS by means of BEB-triggered nanozyme launch and PDA-mediated scavenging, attaining homeostasis to concurrently improve antibacterial motion and assist tissue regeneration (Fig. 3a). H2O2 was used to simulate the impact of excessive ROS environments [60]. In an atmosphere with 2mL 500 µM H2O2, the 300 µL hydrogels degraded quickly inside 10 h (Fig. 3b), with PBPA2 exhibiting the slowest degradation, as a consequence of its greater diploma of crosslinking talked about beforehand. In distinction, in simulated low ROS environments, corresponding to PBS (Fig. S23) or simulated wound environments containing lysozyme (Fig. 3c), confirming its H2O2-responsive launch conduct. This outcomes from oxidation-induced lack of electrophilicity on the boron heart below excessive H2O2, disrupting BEB stability and triggering nanozyme launch [61, 62]. To confirm the discharge of PDA@AgNP from the hydrogel, absorption peak at 576 nm was recognized by means of UV-vis evaluation of various PDA@AgNP concentrations (Fig. S24a). A linear regression mannequin was established based mostly on the absorbance values at 576 nm throughout numerous concentrations, enabling quantitative focus willpower (Fig. S24b). Notably, the hydrogel demonstrated speedy, H2O2-responsive launch of PDA@AgNP nanozymes below excessive H2O2 circumstances (Fig. S24c), according to its degradation profile. Regardless of Fenton-like exercise, radical scavenging dominated the therapeutic consequence, as confirmed by DPPH and NBT assays. PBPA2 exhibited the strongest scavenging capability, evidenced by colorimetric adjustments (Fig. 3d, S25, and S26), with sustained efficacy over 24 h. This highlights PDA’s superior antioxidant position over AgNP radical technology, enabling twin antibacterial and antioxidant regulation. In vitro experiments utilizing DCFH-DA labeling to evaluate ROS ranges additional elucidated the final word antioxidant efficiency in H2O2 induced in vitro mannequin (Fig. 3e). In comparison with the H2O2 group, the PB group, which contained BEB however no PDA, already confirmed ROS depletion, whereas PDA additional enhanced this efficiency. Quantitative move cytometry confirmed the identical outcomes with DCFH-DA fluorescence (Fig. 3f and S27), additional substantiating the ROS responsiveness and scavenging capabilities. Collectively, these outcomes verify that the intrinsic ROS-scavenging capability of BEB and PDA stays efficient even after incorporation of AgNPs, whose nanozyme-generated ROS doesn’t impair general antioxidant efficiency. This establishes a dependable foundation for in vivo utility.
As native ROS concentrations lower in a wound, it facilitates cell migration and proliferation in later phases, selling wound therapeutic. Thus, experiments on keratinocyte migration inhibition by H2O2 with totally different hydrogels have been carried out. H2O2 considerably inhibited migration, with therapy utilizing PBPA1 failing to revive migration to baseline ranges. Solely the introduction of PBPA2 resulted in migration ranges similar to the unfavourable management (Fig. 3g and h). So, each satisfactory BEB crosslinking and ample PDA are important for re-epithelialization. PBPA hydrogels considerably mitigated the harm brought on by H2O2 to keratinocytes and fibroblasts guaranteeing their survival and proliferation (Fig. 3i and okay). Though different hydrogels have been in a position to cut back H2O2 harm to some extent, they may not obtain the wonderful efficiency exhibited by PBPA2 (Fig. S28).
In abstract, the ROS-responsive BEB in PBPA senses an infection and triggers on-demand launch of antibacterial AgNP nanozymes, which generate Ag+ and radicals. In contrast to typical ion-leaching nanobioceramics, this mechanism avoids long-term metallic accumulation and cytotoxicity [63]. Subsequent PDA scavenging additional refines ROS administration, enabling twin management of micro organism and oxidative stress, a programmed technique that achieves ROS-balanced remedy.
ROS-depend clever supply and ROS-scavenger properties of the hydrogels. a) Schematic picture of ROS-depend degradation and cargo-release of the PBPA hydrogel; b) Hydrogel degradation charge in 500 µM H2O2 atmosphere, n = 3; c) Hydrogel degradation charge in 1 mg/ml lysozyme, n = 3; d) Consultant photographs of DPPH and NBT take a look at of various hydrogel; e) Intracellular ROS-scavenger efficiency of various hydrogels by DCFH-DA take a look at on RAW264.7 cell line below H2O2 stimulation, scale bar = 200 μm; f) Flowcytometry of DCFH-DA labeled RAW264.7 cell in fluorescein isothiocyanate FITC-A channel on totally different hydrogels; g) Consultant photographs of HaCaT migration below H2O2 atmosphere treating with totally different hydrogels, scale bar = 100 μm; h) Quantitative evaluation of HaCaT migration, comparability was made to C group, n = 3; i) Consultant photographs of stay/useless staining of HaCaT and NIH3T3 cell below H2O2 atmosphere treating with or with out PBPA2 hydrogel, scale bar = 50 μm; j) Quantitative evaluation of stay/useless staining of HaCaT below H2O2 atmosphere treating with totally different hydrogels, n = 3; okay) Quantitative evaluation of stay/useless staining of NIH3T3 below H2O2 atmosphere treating with totally different hydrogels, n = 3. Knowledge represented imply ± SD. **** p < 0.0001, *** p < 0.001, ** p < 0.01, * p < 0.05
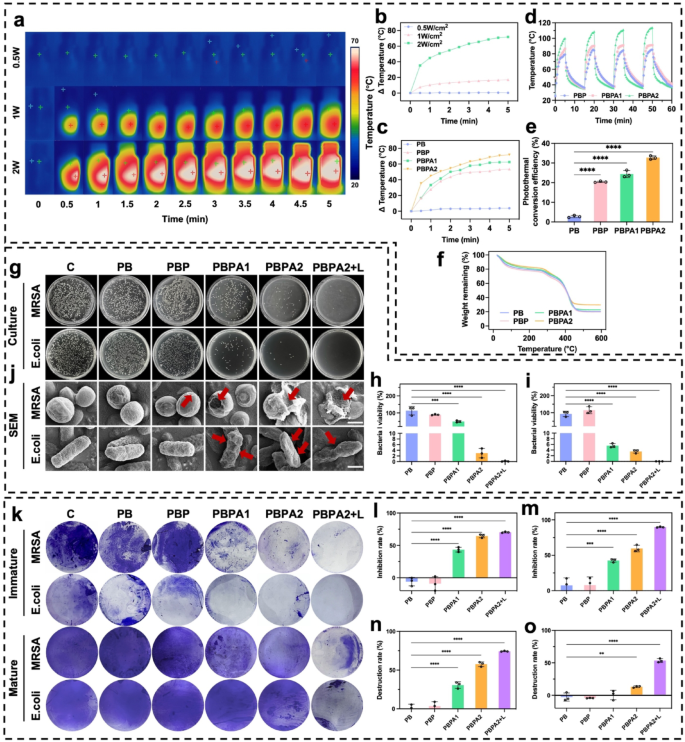
Photothermal means, biofilm disruption, and antibacterial impact of PBPA hydrogel
AgNP integration enhances the PCE of PDA, markedly bettering biofilm disruption and enabling environment friendly AgNP supply. Efficient biofilm eradication facilitates the transition from irritation to proliferation, whereas localized thermal exercise promotes collagen transforming and accelerates pores and skin regeneration [64]. This constitutes the essential step within the general therapeutics.
PB exhibited no photothermal results below any irradiation (Fig. S29). Upon the addition of PDA, a rise in temperature was noticed (Fig. S30). After including AgNP, the rise in temperature turned extra pronounced (Fig. S31), according to outcomes noticed in aqueous programs, confirming the improved PCE by means of AgNP deposition. PBPA2 displayed the strongest photothermal efficiency (Fig. 4a and b), with superior clear distinctions noticed throughout 1 W/cm² (Fig. S32) and a couple of W/cm² (Fig. 4c) NIR irradiation. Moreover, PBPA2 additionally demonstrated speedy heating-cooling capabilities, permitting for efficient reuse throughout 4 cycles inside 1 h below each 1 W/cm² and a couple of W/cm² NIR irradiation (Fig. S33 and 4 d). PBPA2 exhibited the best PCE (Fig. 4e), attributable to AgNP enhancement and elevated PDA@AgNP content material. It additionally demonstrated superior thermal stability (Fig. 4f), enabling optimum NIR responsiveness, environment friendly warmth technology, greater peak temperatures, and constant efficiency, making it best for speedy, repeatable, and steady photothermal biofilm disruption.
Photothermal impact, synthetic triggered biofilm disruption, and antibacterial impact of various hydrogels. a) Consultant photographs of PBPA2 hydrogels below totally different energy of 808 nm NIR; b) Quantitative evaluation of the photothermal impact of PBPA2 hydrogels below totally different energy of 808 nm NIR; c) Quantitative evaluation of the photothermal impact of various hydrogels below 2 W/cm2 808 nm NIR; d) Photothermal stability below repeated on-and-off 808 nm NIR of various hydrogels; e) PCE of various hydrogels, n = 3; f) Photothermal stability of TGA curves of various hydrogels; g) Consultant photographs of colonization of MRSA and E. coli below totally different hydrogel therapies; h) Quantitative MRSA bacterial viability from colonization after totally different hydrogel therapies, n = 3; i) Quantitative E. coli bacterial viability from colonization after totally different hydrogel therapies, n = 3; j) Consultant SEM photographs of MRSA and E. coli below totally different hydrogel therapies, scale bar = 500 nm, pink arrow signifies the destruction; okay) Consultant photographs of the immature biofilm inhibition and mature biofilm destruction below totally different hydrogel therapies stained by crystal violet; l) Quantitative evaluation of immature MRSA biofilm inhibition after totally different hydrogel therapies, n = 3; m) Quantitative evaluation of immature E. coli biofilm inhibition after totally different hydrogel therapies, n = 3; n) Quantitative evaluation of mature E. coli biofilm destruction after totally different hydrogel therapies, n = 3; o) Quantitative evaluation of mature MRSA biofilm destruction after totally different hydrogel therapies, n = 3. Knowledge represented imply ± SD. **** p < 0.0001, *** p < 0.001, ** p < 0.01, * p < 0.05
Antibacterial efficacy towards Escherichia coli (E. coli) and methicillin-resistant Staphylococcus aureus (MRSA) was assessed through plate tradition. AgNP-containing hydrogels alone considerably inhibited bacterial development (Fig. 4g and that i), however the utility of photothermal results led to just about 100% bacterial elimination. SEM confirmed disruption of bacterial morphology below totally different hydrogel therapies (Fig. 4j) and micro organism destruction by PDA@AgNP (Fig. S34). The mixed antibacterial and ROS-scavenging capabilities of PBPA enhance the wound microenvironment, shorten the inflammatory section, and promote development to proliferation. Whereas AgNPs successfully inhibit planktonic micro organism and immature biofilms (Fig. 4okay and m and S35), NIR-induced photothermal disruption is important to eradicate mature biofilms (Fig. 4okay and S36). In circumstances biofilms matured, the standalone AgNPs was inadequate for overcoming the biofilm. Regardless of the flexibility of PBPA2 to disrupt mature biofilms fashioned by E. coli (Fig. 4n), its efficacy in treating MRSA biofilms was not evident with out NIR publicity (Fig. 4o). NIR irradiation is important for PBPA2 to disrupt mature biofilms, serving because the crucial preliminary step that permits subsequent therapeutic efficacy.
In abstract, PBPA2 displays distinctive and reusable photothermal properties, enabling almost 100% bacterial inhibition and offering a good microenvironment. It potently suppresses immature biofilm formation and, below NIR irradiation, robustly disrupts mature biofilms. This photothermal initiation opens therapeutic pathways for subsequent nanozyme supply, making it extremely appropriate for sensible functions.
In vitro anti-inflammation impact and biocompatibility of PBPA hydrogel
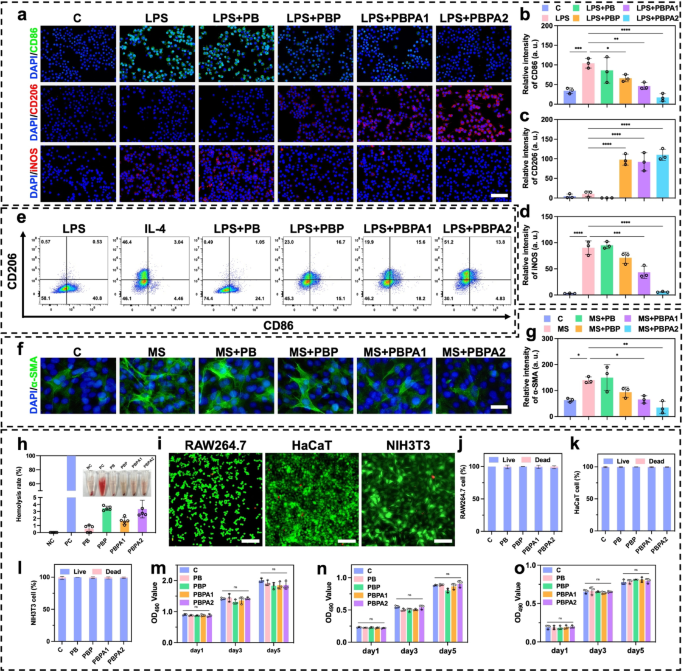
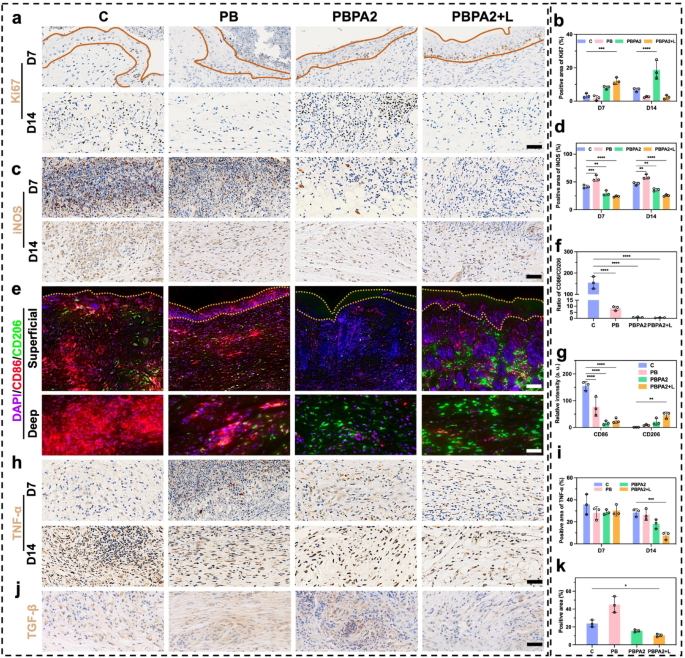
Earlier experiments verified that PBPA mitigate oxidative stress abnormalities brought on by excessive concentrations of H2O2 (Fig. 3e). In contaminated wounds, the pathological microenvironment options excessive ROS and LPS exercise. LPS drives M1 macrophage polarization and extreme ROS technology, which the PBPA hydrogel successfully attenuates, most notably with greater PDA loading (Fig. S37). This suppression is attributed to PDA-derived dopamine and quinone, which mitigate LPS-induced irritation [65]. LPS-induced ROS overproduction primarily stems from M1 polarization, as proven by elevated CD86 (Fig. 5a and b) and iNOS (Fig. 5a and d) expression [66]. In distinction, M2 polarization marked by elevated CD206 (Fig. 5a and c) expression is considerably suppressed. PBPA therapy successfully reprograms this LPS-driven polarization sample towards anti-inflammatory M2 phenotypes (Fig. 5a and d). Notably, PDA incorporation not solely reverses LPS-induced M1 polarization but additionally achieves ranges similar to interleukin (IL)−4-induced constructive controls (Fig. 5e).
LPS exacerbates irritation in NIH3T3 fibroblasts [67] and upregulates α-SMA expression in NIH3T3 cells [12], potentiating everlasting myofibroblast activation and pathological scarring [49]. Earlier research show that LPS-stimulated macrophage-fibroblast co-cultures considerably elevate TGF-β expression [13], which serves as the first upstream regulator of α-SMA. Utilizing conditioned medium from LPS-polarized macrophages (MS) to deal with NIH3T3 successfully fashions this macrophage-driven fibrotic response [66], capturing α-SMA induction through inflammatory-proliferative crosstalk. Key findings reveal that MS-cultured NIH3T3 cells exhibit elevated α-SMA expression (Fig. 5f and g). In keeping with anti-inflammatory results on macrophages in vitro, PDA considerably attenuates fibrotic responses, with PBPA2 exhibiting the strongest efficacy, confirming that PBPA2 modulates macrophage conduct to downstream suppress α-SMA expression and myofibroblast activation.
Biocompatibility and toxicity are crucial for medical translation. Hemolysis assays confirmed that every one hydrogels exhibited hemolysis charges under 5% when cocultured with blood (Fig. 5h). In vitro biocompatibility was assessed utilizing RAW264.7 macrophages, HaCaT keratinocytes, and NIH3T3 fibroblasts. Reside/useless staining after 24-hour coculture confirmed good compatibility (Fig. 5i and l and S38). Furthermore, five-day steady publicity to hydrogels induced no vital toxicity (Fig. 5m and o).
The PBPA hydrogel modulates M1 polarization below an infection, selling a shift towards M2 phenotypes to shorten the inflammatory section. It additionally suppresses LPS- and M1-induced fibroblast overactivation, decreasing fibrotic scarring. Moreover, the hydrogel demonstrates wonderful biocompatibility with out detectable cytotoxicity in vitro.
In vitro anti-inflammation impact and biocompatibility of various hydrogels. a) Immunofluorescence staining of CD86, CD206, and iNOS on RAW 264.7 below totally different therapy, scale bar = 100 μm; b) Relative depth of CD86 based mostly on immunofluorescence staining, n = 3; c) Relative depth of CD206 based mostly on immunofluorescence staining, n = 3; d) Relative depth of iNOS based mostly on immunofluorescence staining, n = 3; e) Circulation cytometry evaluation of CD206 and CD86 on RAW 264.7 below totally different therapy; f) Immunofluorescence staining of α-SMA on NIH3T3 below totally different therapy, scale bar = 100 μm; g) Relative depth of α-SMA based mostly on immunofluorescence staining, n = 3; h) Quantitative evaluation and consultant photographs of the hemolysis take a look at after treating with totally different hydrogels, n = 3; i) Consultant photographs of stay/useless staining of RAW264.7, HaCaT, and NIH3T3 handled with PBPA2 hydrogel, scale bar = 50 μm; j) RAW264.7, okay) HaCaT, and l) NIH3T3 stay/useless staining quantitative evaluation after treating with totally different hydrogels, n = 3; m) RAW264.7, n) HaCaT, and o) NIH3T3 MTT take a look at quantitative evaluation after treating totally different hydrogels at corresponding time level, n = 3. Knowledge represented imply ± SD. **** p < 0.0001, *** p < 0.001, ** p < 0.01, * p < 0.05
PBPA accelerating regenerative contaminated wound therapeutic in vivo
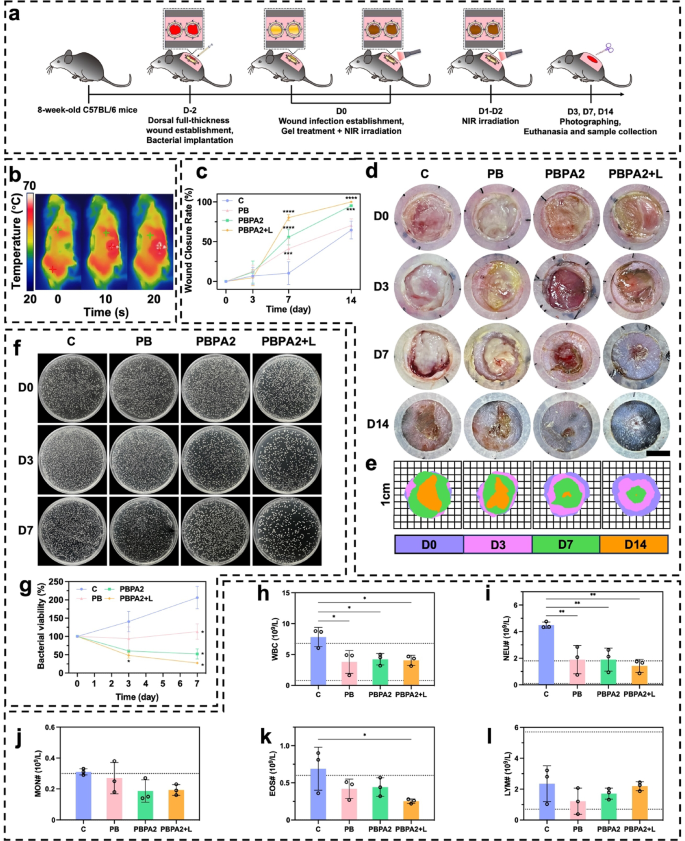
PDA@AgNP achieves a singular steadiness between antibacterial exercise and ROS modulation, not like metal-oxide nanozymes (e.g., CeO2), which exhibit sturdy catalysis however restricted photothermal effectivity and flexibility. By integrating the peroxidase-like exercise of AgNPs with PDA’s antioxidant and photothermal capacities, PDA@AgNP allows concurrent biofilm disruption, bacterial eradication, and ROS clearance. This twin performance stabilizes AgNPs towards oxidation and permits programmable catalytic response, outperforming typical single-function nanozymes in biofilm-infected wound therapy. To judge sensible efficiency, MRSA and E. coli biofilm-infected fashions have been established 48 h previous to therapy. Experiments commenced as soon as a yellowish membrane confirmed biofilm formation (Fig. 6a). Photothermally triggered biofilm disruption achieved the required temperature inside simply 10 s (Fig. 6b and S39). On D3 of therapy, though wound space confirmed no vital distinction amongst teams (Fig. 6c), the PBPA2 + L group displayed markedly lowered redness, minimal exudate, and no swelling (Fig. 6d and e), indicating near-complete eradication of biofilm and micro organism, together with profitable native ROS consumption, according to the ROS-responsive antimicrobial launch. This early intervention laid the groundwork for accelerated therapeutic, with PBPA2 + L attaining optimum wound closure charges of 80.27% (D7) and 99.92% (D14) (Fig. 6c and e). Critically, PBPA2 + L considerably lowered early exudation and hemorrhage, limiting eschar formation; by D14, substantial eschar was noticed in all different teams besides PBPA2 + L (Fig. 6d). Management experiments confirmed that therapeutic advantages originated from photothermal activation somewhat than NIR irradiation alone (Fig. S40). Bacterial tradition of wound exudate on D3 and D7 demonstrated sturdy antibacterial efficacy (Fig. 6f and g). Moreover, blood checks on D14 confirmed that the C group nonetheless exhibited elevated white blood cell (WBC) ranges, indicating ongoing an infection (Fig. 6h), primarily characterised by elevated neutrophil ranges (Fig. 6i and l). Though the PB and PBPA2 teams confirmed slight reductions in neutrophils, ranges remained above regular, underscoring that solely PBPA2 + L remedy achieved full therapeutic effectiveness.
In vivo utility of various therapy for biofilm-infected wound. a) Schematic illustration of the process for biofilm an infection mannequin institution and programmed therapy. b) Consultant infrared photographs of the photothermal impact of PBPA2 hydrogel on mice; c) Quantitative evaluation of wound therapeutic charge below totally different therapies, n = 3; d) Consultant photographs on totally different time factors of biofilm-infected wounds handled with totally different therapies, scale bar = 4 mm; e) Schematic photographs on totally different time factors of biofilm-infected wounds handled with totally different therapies; f) Consultant photographs of colonization of micro organism on wound exudate at decided time level after totally different therapies; g) Quantitative evaluation of corresponding bacterial colonization, n = 3; h) White blood cell counting of blood samples from mice handled with totally different therapies, n = 3; i) Neutrophil counting of blood samples from mice handled with totally different therapies, n = 3; j) Monocyte counting of blood samples from mice handled with totally different therapies, n = 3; okay) Eosinophil counting of blood samples from mice handled with totally different therapies, n = 3; l) Lymphocyte counting of blood samples from mice handled with totally different therapies, n = 3. Knowledge represented imply ± SD. **** p < 0.0001, *** p < 0.001, ** p < 0.01, * p < 0.05
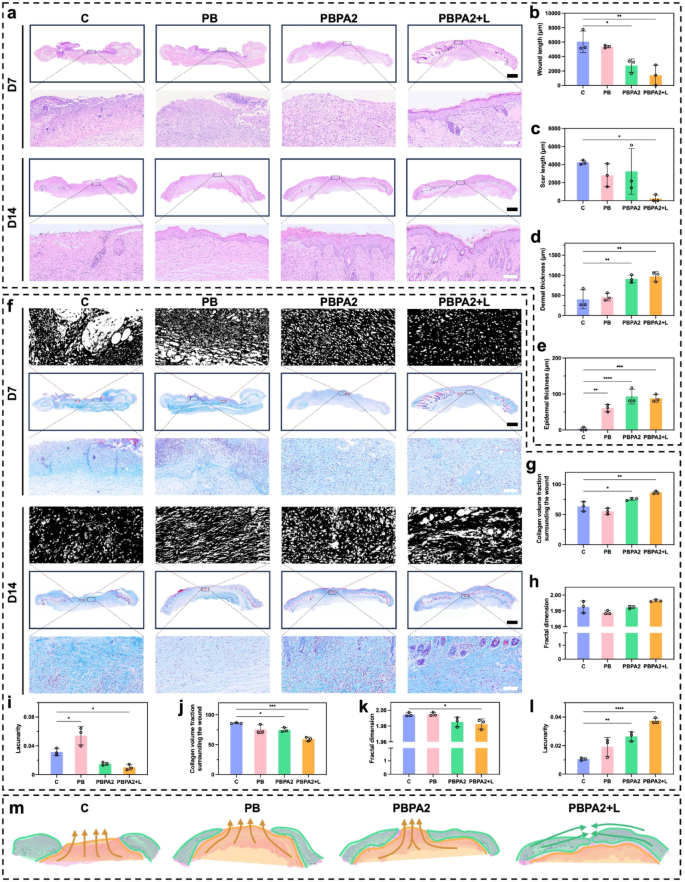
H&E staining was used for histological evaluation of wound therapeutic (Fig. 7a). On D7, vital wounds remained within the C and PB teams, whereas hydrogel therapies promoted granulation tissue formation and elevated dermal thickness (Fig. 7b and d). Nonetheless, solely below the PBPA2 + L therapy was speedy re-epithelialization on D7 (Fig. 7a), according to gross observations (Fig. 6d). These observations counsel that on D7, the C and PB teams remained within the hemostatic-inflammatory section, whereas teams handled with PBPA2, with or with out gentle, had superior to the proliferative-remodeling section. Fast re-epithelialization, as seen within the PBPA2 + L group, reduces the danger of hypertrophic scarring and helps regenerative therapeutic [64]. By D14, solely the C group displayed non-healing wounds with persistent an infection and eschar. Though different teams achieved full re-epithelialization (Fig. 7e), however the PB and PBPA2 teams confirmed vital scarring (Fig. 7c), characterised by lowered native pores and skin appendages and in depth granulation tissue fibrosis. This means that PBPA2 + L considerably inhibits scar formation and enhances pores and skin regeneration.
Masson’s staining and fractal evaluation revealed extra ample and arranged collagen deposition within the PBPA2 + L group by D7 (Fig. 7f and g), indicative of superior proliferative section transition and a pro-regenerative fibroblast microenvironment. Quantitatively, programmed therapy led to a extra two-dimensional collagen structure (Fig. 7h) and decrease lacunarity (Fig. 7i), reflecting superior matrix group. Nonetheless, this end result reversed by D14. The C group ultimately initiated fibroblast proliferation and collagen secretion by means of intrinsic therapeutic mechanisms, whereas handled teams, particularly PBPA2 + L, exhibited looser collagen distribution, indicating lively transforming (Fig. 7f). Fractal evaluation confirmed sparser collagen in PBPA2 + L, attribute of mature transforming that reduces mechanical rigidity and creates house for pores and skin appendage regeneration. Quantitatively, PBPA2 + L displayed the bottom collagen density (Fig. 7j). This can be as a consequence of fibroblasts secreting a provisional, fibrin-rich ECM somewhat than a collagen-rich one, thus selling scarless and speedy wound therapeutic [49]. Moreover, collagen distribution was additional removed from a two-dimensional construction (Fig. 7okay), and lacunarity was greater (Fig. 7l), offering transforming house for pores and skin appendage regeneration and decreasing native rigidity.
These outcomes counsel that PBPA2 + L therapy advances the development of the therapeutic phases, shortening the inflammatory section and quickly transitioning to the proliferative section to advertise swift wound therapeutic. Moreover, below the excellent administration of multi-modal therapies, the native construction turns into extra relaxed, decreasing tension-induced scar formation and facilitating the shift from a restore mode to a regeneration mode.
Apparently, variations of therapeutic sample between PBPA2 + L group and different teams have been noticed (Fig. 7m), as evidenced by a predominantly peripheral-to-central therapeutic sample, with much less granulation tissue from backside up, an identical morphology of therapeutic heart to the encircling tissue, and an accelerated re-epithelialization. In distinction, different teams have been principally full of granulation tissue backside up. This means the therapy shifted therapeutic sample to “peripheral-to-central” somewhat than “bottom-to-up”, which offer foundation for subsequent transforming and pores and skin regeneration. To confirm this speculation, orientation-related analyses is required. Nonetheless, there’s presently no appropriate technique in pores and skin. Along with the beforehand reported scar patterns [68], a brand new analytical technique in following part that spatially divides the pores and skin based mostly on the therapeutic outcomes and analyses every area individually was proposed within the current examine to show that programmed therapy can enhance regeneration effectivity by directing therapeutic orientation.
H&E and Masson’s staining of pores and skin part for analysis of pores and skin regeneration. a) H&E staining of pores and skin on D7 and D14 in numerous therapy group, scale bar of entire part = 1 mm, scale bar of enlarged half = 100 μm; b) Wound size on D7 calculated by means of H&E staining, n = 3; c) Scar size on D14 calculated by means of H&E staining, n = 3; d) Dermal thickness on D7 calculated by means of H&E staining, n = 3; e) Epidermal thickness on D14 calculated by means of H&E staining, n = 3; f) Masson’s staining on D7 and D14 in numerous therapy group with fractal photographs, scale bar of entire part = 1 mm, scale bar of enlarged half = 100 μm; g) Collagen quantity fraction surrounding wounds of D7 calculated by means of Masson’s staining, n = 3; h) Fractal dimension calculated on wound space of D7 by means of Masson’s staining, n = 3; i) Lacunarity calculated on wound space of D7 by means of Masson’s staining, n = 3; j) Collagen quantity fraction surrounding scars of D14 calculated by means of Masson’s staining, n = 3; okay) Fractal dimension calculated on scar space of D14 by means of Masson’s staining, n = 3; l) Lacunarity calculated on scar space of D14 by means of Masson’s staining, n = 3; m) Therapeutic orientation sample distinction amongst teams (orange signifies “bottom-to-top mobilized fascia scar filling mode”, inexperienced signifies “peripheral-to-central regular tissue creeping restore mode”). Knowledge represented imply ± SD. **** p < 0.0001, *** p < 0.001, ** p < 0.01, * p < 0.05
Therapeutic orientation adjustments after the therapy revealed by patch restore division technique evaluation
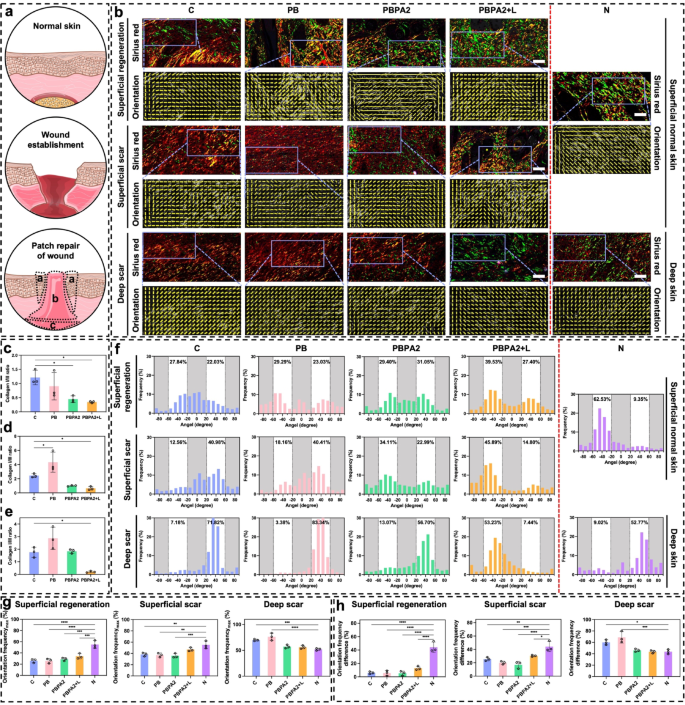
Wound therapeutic is a mobilized patch restore of the underlying fascia, resembling a volcanic crater (Fig. 8a) [68]. To raised analyze spatial therapeutic patterns, this examine introduces the Patch Restore Division Methodology, which divides the wound-scar/regeneration zone into three distinct areas (Fig. 8a). Superficial Regeneration Space (a): Ensuing from centripetal migration of surrounding dermal and epidermal tissues, this area usually displays near-normal construction with pores and skin appendages and lies throughout the unique wound boundary exterior the central scar. Superficial Scar Space (b): Shaped by speedy upward closure from underlying fascia and granulation tissue, this central area lacks typical pores and skin structure and performance. Deep Scar Space (c): Originating from deep mobilized fascia that migrates upward, this space varieties the bottom of the central scar. These areas differ basically in location, extent, orientation, and microstructure of restore. To check the speculation that programmed therapy alters therapeutic orientation, every area was in comparison with corresponding regular pores and skin areas alongside directional evaluation, enabling exact analysis of regenerative outcomes.
Throughout all three areas, the PBPA2 + L group confirmed essentially the most pronounced inexperienced staining, indicating a sort III collagen-rich therapeutic sample (Fig. 8b and e). A lowered kind I/III collagen ratio signifies decrease scarring threat and displays distinct ECM transforming dynamics. Wholesome transforming sometimes options early kind III collagen deposition, later step by step changed by kind I collagen, a key indicator of wound maturity. Unexpectedly, teams with poorer outcomes exhibited greater kind I collagen, whereas the best-healing group (PBPA2 + L) maintained a sort III-dominant profile. This means that though all teams reached superior ECM transforming levels, PBPA2 + L uniquely preserved excessive kind III collagen ranges, accelerating therapeutic whereas minimizing scar formation.
Picro-Sirius Pink staining and therapeutic orientation variations amongst three distinct therapeutic elements. a) Schematic illustration of wound therapeutic and the three totally different native therapeutic space (a: Superficial regeneration-indicating full regeneration above dermal space; b: Superficial scar-indicating scar formation above dermal space; c: Deep scar-indicating scar formation under dermal space); b) Consultant photographs of Picro-Sirius Pink staining and orientation photographs on D14 in numerous therapy teams examine to regular pores and skin based mostly on the three totally different therapeutic space, scale bar = 50 μm; c) Collagen I/III ratio of superficial regeneration space, n = 3; d) Collagen I/III ratio of superficial scar space, n = 3; e) Collagen I/III ratio of deep scar space, n = 3; f) Orientation distribution of various therapy teams examine to regular pores and skin based mostly on the three totally different therapeutic space; g) Maximal single orientation frequency amongst totally different teams in three distinct areas, n = 3; h) Frequency distinction between two totally different orientations amongst totally different teams in three distinct areas, n = 3. Knowledge represented imply ± SD. **** p < 0.0001, *** p < 0.001, ** p < 0.01, * p < 0.05
Within the superficial regeneration space of every group, though regeneration appeared related in gross construction, the C group exhibited a extra disorganized association, and solely the PBPA2 + L group achieved regeneration predominantly with kind III collagen (Fig. 8b). Moreover, irritation or different undetected elements influenced the section of collagen secretion. In group C, collagen I used to be predominant, whereas collagen III was extra outstanding below PBPA2 + L remedy. Though orientation distribution didn’t present vital enchancment, it was highest on this area below PBPA2 + L therapy at 39.53% (Fig. 8f), indicating a extra polarized therapeutic sample. Provided that this area is positioned within the superficial construction, its polarity originated from the periphery somewhat than the underlying fascia. This means that the PBPA2 + L therapy promotes the peripheral regular tissue to regenerate in the direction of the middle, somewhat than the dense granulation tissue mobilized from the fascia beneath, thereby exhibiting greater distribution polarity.
Staining indicated that, apart from the PBPA2 + L group, the restore mode in different teams was dominated by kind I collagen-based patch restore, characterised by dense collagen construction within the superficial scar space. Solely the PBPA2 + L group had a unfastened construction much like the superficial pores and skin of the N group (Fig. 8b). Furthermore, the orientation distribution polarity at this website reached a most of 45.89% following PBPA2 + L therapy (Fig. 8f). These outcomes counsel that within the central area of the wound, the gradual section transition and exacerbation of irritation limit the peripheral-to-central creeping boundary in teams apart from the PBPA2 + L therapy. This restriction forces the dense mobilized fascia under to fill the hole upward, resulting in the whole formation of scars incapable of regenerating regular buildings. This bidirectional therapeutic reduces distribution polarity. Nonetheless, after PBPA2 + L therapy, this limitation is overcome, permitting a horizontal creeping sample of therapeutic from the periphery in the direction of the middle to dominate. This ends in a distribution polarity at this stage and a porous construction resembling regular pores and skin, somewhat than the dense construction typical of patch restore. Though the distribution of collagen kind III on this space isn’t as optimum as within the superficial regeneration space below PBPA2 + L therapy, it stays the most effective among the many teams. This demonstrates the success of the therapy in changing scar formation with pores and skin regeneration.
The variations have been extra evident within the deep scar space. Orientation evaluation prompt that, whereas the collagen orientation in all teams primarily exhibited a excessive single orientation distribution, the C and PB teams displayed an especially excessive single orientation of 70%−80%, indicating the migration and restore of the wound crater by patch restore. This symmetry with the superficial space ends in the deeper mobilized fascia within the C and PB teams making an attempt to fill upwards, resulting in a a lot greater single orientation distribution. In distinction, below PBPA2 + L therapy, the therapeutic sample is dominated by an outside-in strategy. This inhibits the migration of mobilized fascia within the deeper layers, leading to a major lower in orientation distribution polarity. The PBPA2 + L group’s distribution decreased to a stage similar to regular pores and skin at round 50% (Fig. 8b and f).
Lastly, a quantitative evaluation of the orientation distribution was carried out to judge variations in collagen alignment. The tissue was divided into three areas for evaluation: superficial regeneration, superficial scar, and deep scar. First, the utmost values of the orientation distribution (Orientation distributionMax) from Fig. 8f have been quantified (Fig. 8g). Amongst all three areas, the PBPA2 + L group exhibited a distribution closest to that of regular pores and skin, with essentially the most pronounced enchancment noticed within the deep scar space, the place PBPA2 utility alone already yielded values approaching these of regular tissue. Solely the C and PB teams confirmed considerably greater values, according to the established position of deep fascia mobilization in scar formation. Whereas the Orientation distributionMax displays polarized alignment traits, it doesn’t totally seize the general structural group. To raised signify structural adjustments, the distinction between the excessive and low polarization values was calculated (Fig. 8h). Though all teams nonetheless differed notably from Group N within the superficial regeneration space, the PBPA2 + L group achieved the best worth among the many handled teams. Ends in different areas aligned with these in Fig. 8g, displaying gentle enchancment in superficial scar and, most notably, suppression of scar-prone deep fascia mobilization to a stage similar to regular pores and skin. These findings show the sturdy scar-modulating capability of the therapy.
General, within the superficial regeneration space, there was a predominantly peripheral-to-central crawling therapeutic sample, with all teams exhibiting pores and skin regeneration. Consequently, every orientational distribution group confirmed an identical sample at this website. Nonetheless, the PBPA2 + L group had the best single polarity, demonstrating essentially the most singular directionality of therapeutic on this space. This means that the inward migration was essentially the most pronounced, serving as vital proof for the shortage of scar development within the ultimate heart. Within the superficial scar space, there was a major distinction between the staining outcomes among the many teams. Solely the PBPA2 + L group confirmed a great construction, correlating with the excessive polarity of its peripheral superficial regeneration space. Photographs from all different teams confirmed no regular dermal construction and a lower in distribution polarity, possible as a result of neutralization of the twin therapeutic sample of bottom-to-up and peripheral-to-central on this space. This modification was evident within the deep scar space, the place distribution polarity was excessive within the first two teams and fell again to round 50% after PBPA2 therapy. This means that migration within the deep space was drastically lowered, exactly as a result of the peripheral-to-central migration within the superficial space was ample for wound therapeutic with out the necessity for deep tissue involvement. Taken collectively, the conclusion that PBPA2 + L therapy resulted in a change in spatial therapeutic orientation was confirmed by Patch Restore Division Methodology evaluation.
In vivo anti-inflammation results of the therapies
The PBPA2 + L therapy promoted a peripheral-to-central migration sample within the superficial space, intently linked to accelerated re-epithelialization. To judge keratinocyte proliferation on the wound edge, immunohistochemical staining for Ki67, a key proliferation marker, was carried out. Outcomes demonstrated considerably elevated Ki67 expression within the basal layer of the dermis in each PBPA2 and PBPA2 + L therapy teams at D7 (Fig. 9a and b). By D14, the PBPA2 group exhibited the best Ki67 expression within the deep wound area (Fig. 9a and b), according to earlier findings of extreme bottom-to-up patch restore in each superficial and deep scar areas. The proliferative surge correlated with localized neo-epidermal thickening, indicating Ki67-high disruptive hyperplasia somewhat than organized stratification. In distinction, the PBPA2 + L group confirmed moderated Ki67 and a thinner, extra uniform dermis, according to enhanced keratinocyte migration, accelerated wound closure, and well timed transforming. Earlier in vitro information indicated the therapy alters fibroblast-to-myofibroblast and M1-to-M2 macrophage differentiation, decreasing reliance on myofibroblast-driven “bottom-up” dermal filling and selling a looser, slower-growing basal construction. To additional elucidate these mechanisms, following experiments have been carried out.
At D7, substantial inflammatory cell infiltration, together with macrophages, continued within the central wound space. To judge this, iNOS expression was examined within the mid-to-deep wound areas. Notably, the C and PB teams, missing PDA-mediated anti-inflammatory/antioxidant remedy and AgNP-based antibacterial results, confirmed considerably greater iNOS expression in comparison with the latter two therapy teams, a development that continued till D14 (Fig. 9c and d). Provided that iNOS displays each irritation and M1 macrophage polarization, twin immunofluorescence staining for CD86 and CD206 was carried out to validate the in vitro findings of PBPA-induced M2 polarization (Fig. 9e). By D14, the extreme M1 polarization shifted towards M2 polarization throughout all teams, attributable to ROS scavenging by PB, the mixed anti-inflammatory and antibacterial results of PBPA2, and the disruption of biofilm obstacles by PBPA2 + L remedy. This shift was evident in each the M1/M2 ratio (Fig. 9f) and expression ranges of M1 and M2 markers (Fig. 9g). Concurrently, tumor necrosis factor-α (TNF-α) ranges at D14 additional confirmed lowered irritation following PBPA2 + L therapy (Fig. 9h and that i). As beforehand noticed, PBPA2 + L remedy successfully suppressed M1 polarization whereas selling M2 polarization by means of twin antimicrobial and anti inflammatory regulation, thereby changing the native pro-inflammatory microenvironment into an anti-inflammatory one. This anti-inflammatory milieu possible contributed to lowered activation of myofibroblasts, aligning with the noticed lower in TGF-β expression within the PBPA2 + L group at D14 (Fig. 9j and okay). Moreover, earlier in depth proof signifies that efficient suppression of irritation, exemplified right here by the downregulation of iNOS and TNF-α, is important for advancing the wound from the inflammatory to the proliferative section. In parallel, polarization towards the M2 phenotype not solely reinforces this transition by means of anti-inflammatory cytokines corresponding to interleukin-10, but additionally offers pro-regenerative signals-VEGF and EGF-that stimulate fibroblast proliferation, collagen deposition, and neovascularization [69]. When coupled with the altered therapeutic orientation induced by PBPA2 + L, these cues facilitate an orderly shift from proliferation into transforming, guaranteeing that matrix deposition and tissue structure evolve towards regeneration somewhat than fibrotic restore.
Collectively, these findings show that the PBPA2 nanozyme hydrogel therapeutic system mitigates the danger of post-infection inflammatory exacerbation within the wound microenvironment, thereby facilitating a phenotypic shift from M1 to M2 macrophage polarization. These findings have been according to in vitro outcomes. This reprogrammed macrophage inhabitants subsequently modulates downstream myofibroblast activation pathways, exhibiting the potential to suppress extreme TGF-β expression. The noticed attenuation of native fibrosis and lowered Ki67 expression within the epidermal layer could collectively contribute to the transition from patch restore to a peripheral-to-central therapeutic sample. Ideally, the peripheral-to-central therapeutic sample may generate a tissue structure extra intently resembling native dermal-epidermal group, doubtlessly yielding considerably improved pores and skin regeneration outcomes in comparison with deep patch restore. To judge the useful penalties of this therapeutic orientation shift, subsequent validation was carried out specializing in pores and skin appendage regeneration and fibrosis parameters.
Immunofluorescence and immunohistochemistry staining of pores and skin part below totally different therapy. a) Immunohistochemistry staining of Ki67 (space inside yellow strains signifies dermis), scale bar = 100 μm; b) Constructive space of Ki67 based mostly on immunohistochemistry staining, n = 3; c) Immunohistochemistry staining of iNOS, scale bar = 100 μm; d) Constructive space of iNOS based mostly on immunohistochemistry staining, n = 3; e) Immunofluorescence staining of CD86 and CD206 (space inside yellow strains signifies dermis), higher scale bar = 200 μm, decrease scale bar = 100 μm; f) Ratio of CD86/CD206 based mostly on immunofluorescence staining, n = 3; g) Relative depth of CD86 and CD206 based mostly on immunofluorescence staining, n = 3; h) Immunohistochemistry staining of TNF-α, scale bar = 100 μm; i) Constructive space of TNF-α based mostly on immunohistochemistry staining, n = 3; j) Immunohistochemistry staining of TGF-β on D14, scale bar = 100 μm; okay) Constructive space of TGF-β based mostly on immunohistochemistry staining, n = 3. Knowledge represented imply ± SD. **** p < 0.0001, *** p < 0.001, ** p < 0.01, * p < 0.05
Pores and skin regeneration improved after therapeutic orientation adjustments
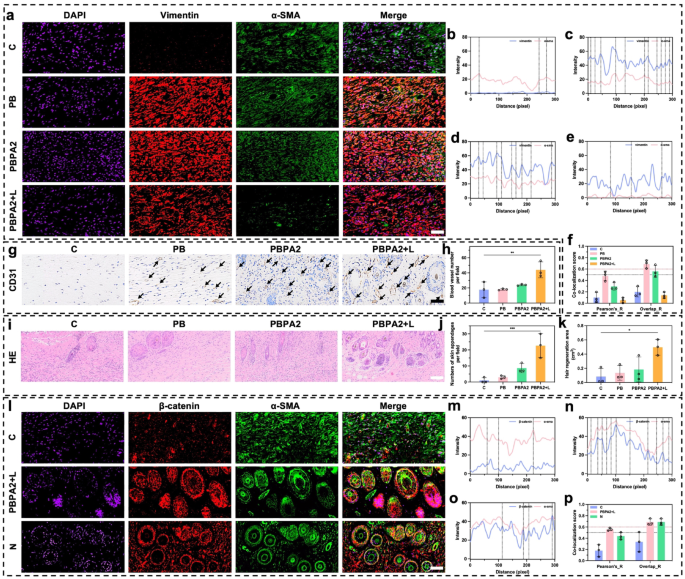
Scar formation is intently linked to myofibroblast activation, marked by α-SMA and vimentin co-expression [70]. This course of primarily arises from patch restore, involving upward migration of deep tissue [68]. For the reason that hair-bearing superficial dermis complicates fibrosis analysis, we centered subsequent evaluation on deep patch restore tissue. Immunofluorescence co-staining revealed maximal myofibroblast abundance at D14 within the PB and PBPA2 teams, which correlated with extreme scarring. In distinction, the C group confirmed weaker vimentin expression, according to its impaired therapeutic. Within the PBPA2 + L group, co-expression of α-SMA and vimentin was considerably decrease (Fig. 10a). Semi-quantitative colocalization evaluation revealed low vimentin expression within the C group (Fig. 10b), excessive fluorescence depth of vimentin and α-SMA within the PB and PBPA2 teams (Fig. 10c and d), and low fluorescence depth and overlapping peaks within the PBPA2 + L group (Fig. 10e). Equally, Pearson’s ratio and Overlap ratio indicated that the PBPA2 + L therapy group had the bottom ranges of fibrotic myofibroblast activation, whereas the teams with pronounced scarring had the best (Fig. 10f and S41). Discount in vimentin could also be as a consequence of a lower within the bottom-to-up therapeutic sample, however, in fact, there are a number of different elements moreover this. First, transient myofibroblasts within the therapy don’t transition to persistent myofibroblasts, thus quickly repairing the wound within the early section and decreasing activation in the course of the transforming section, selling pores and skin regeneration as an alternative of scar formation [49]. Second, additionally it is attainable that clearance of ROS can result in vimentin expression downregulation and decreased downstream TGF-β expression which was confirmed in Fig. 9j [71]. This commentary may additionally correlate with our earlier in vitro findings that PDA@AgNPs exert antibacterial results whereas modulating macrophage polarization, consequently downregulating TGF-β expression in downstream fibroblasts (Fig. 5f). Moreover, lowered vimentin resulted from decreased kind I collagen secretion [71]. Subsequently, the lowered myofibroblast activation resulted in a extra loosely organized tissue construction conducive to transforming. Inside this pro-regenerative microenvironment, considerably upregulated expression of the vascular marker CD31 was noticed (Fig. 10g-h and S42). Such vascular transforming is suggestive of useful neovascularization, which is crucial for restoring microcirculatory assist, guaranteeing satisfactory perfusion and nutrient trade, and thereby sustaining the metabolic calls for of regenerating pores and skin tissue.
For the reason that superficial dermis is the first area for hair follicle regeneration, evaluation on this space to judge pores and skin regeneration outcomes was primarily centered. Following enhanced angiogenesis and structural transforming, a better variety of pores and skin appendages on this layer within the PBPA2 + L group was decided (Fig. 10i and j). Macroscopic evaluation of the regenerated hair space additionally mirrored related outcomes (Fig. 10okay). α-SMA and β-catenin are markers extremely related to hair follicle neogenesis. Co-immunofluorescence staining for α-SMA and β-catenin permits clear commentary of recent hair follicle growth [70]. The PBPA2 + L group exhibited an upregulated β-catenin sign within the superficial dermis in comparison with the C group, and the outcomes have been extremely much like the conventional group (N) (Fig. 10l). Semi-quantitative colocalization evaluation indicated extra overlapping peaks of β-catenin and α-SMA sign within the PBPA2 + L group in comparison with the C group, and much like the N group. Moreover, the fluorescence depth of β-catenin in each teams was greater than within the C group (Fig. 10m and o). Quantitative colocalization indices, together with Pearson’s ratio and Overlap ratio, confirmed that hair follicle neogenesis within the PBPA2 + L group surpassed that of the C group, reaching a stage similar to regular pores and skin (Fig. 10p and S43). The underlying mechanisms are possible multifactorial. Through the transforming section of wound therapeutic, the optimization of tissue structure and enhanced angiogenesis present crucial microenvironmental assist for hair follicle regeneration [72, 73]. As collagen fibers realign and the ECM undergoes dynamic transforming, the initially disorganized scar tissue is progressively changed by extra physiologically useful connective tissue. This structural enchancment not solely reinforces mechanical assist for perifollicular tissues but additionally establishes a extra favorable development area of interest for hair follicle stem cells [74, 75]. Importantly, the β-catenin/α-SMA co-localization noticed right here may aligns with activation of the follicular stem cell area of interest, a prerequisite for initiating new anagen cycles [76]. By reawakening quiescent stem cells inside a supportive microenvironment, the regenerative course of extends past follicle formation to the restoration of useful hair-bearing pores and skin. Concurrently, a sturdy community of nascent capillaries varieties densely below sustained stimulation by development elements corresponding to VEGF, considerably bettering native blood perfusion and nutrient provide. These newly fashioned vessels not solely ship oxygen and important vitamins but additionally transport numerous cytokines and signaling molecules that promote hair follicle activation [77]. Inside this extremely vascularized microenvironment that extra intently resembles native tissue, quiescent hair follicle stem cells are reactivated, exhibiting markedly enhanced proliferative and differentiation capacities that drive follicles into new anagen cycles. Moreover, particular ECM parts, together with laminin and fibronectin secreted by activated fibroblasts throughout transforming, kind a three-dimensional scaffold that helps dermal papilla cell aggregation and follicular morphogenesis, in the end facilitating the regeneration of extra strong hair buildings [78]. The synergistic interaction between tissue transforming and angiogenesis basically reconstructs a follicle microenvironment resembling native tissue, establishing each the structural basis and physiological circumstances essential for useful hair regeneration.
Analysis of pores and skin appendages regeneration and fibrotic scar formation after therapeutic orientation change. a) Immunofluorescence staining of DAPI, vimentin, and α-SMA in deeper dermis on D14, scale bar = 50 μm; b) Immunofluorescence colocalization evaluation of vimentin and α-SMA in management group; c) Immunofluorescence colocalization evaluation of vimentin and α-SMA in PB group; d) Immunofluorescence colocalization evaluation of vimentin and α-SMA in PBPA2 group; e) Immunofluorescence colocalization evaluation of vimentin and α-SMA in PBPA2 group; f) Colocalization index calculated by means of vimentin and α-SMA immunofluorescence staining in numerous teams, black line signifies threshold of Pearson’s ratio which is 0.5, pink line signifies threshold of Overlaps ratio which is 0.6, n = 3; g) Immunochemistry staining of CD31 on D14, black arrows point out blood vessels, scale bar = 100 μm; j) Blood vessel quantity per area calculated on immunochemistry staining, n = 3; i) Pores and skin appendages regeneration in numerous therapy teams on H&E staining, scale bar = 100 μm; j) Variety of pores and skin appendages on D14 per area calculated on H&E staining, n = 3; okay) Space of hair regeneration on D14 of mice, n = 3; l) Immunofluorescence staining of DAPI, β-catenin, and α-SMA in superficial dermis on D14, scale bar = 50 μm; m) Immunofluorescence colocalization evaluation of β-catenin and α-SMA in management group; n) Immunofluorescence colocalization evaluation of β-catenin and α-SMA in PBPA2 + L group; o) Immunofluorescence colocalization evaluation of β-catenin and α-SMA in regular pores and skin; p) Colocalization index calculated by means of β-catenin and α-SMA immunofluorescence staining in numerous teams, black line signifies threshold of Pearson’s ratio which is 0.5, pink line signifies threshold of Overlaps ratio which is 0.6, n = 3. Knowledge represented imply ± SD. **** p < 0.0001, *** p < 0.001, ** p < 0.01, * p < 0.05
These outcomes trace {that a} peripheral-to-central dominant therapeutic sample after the therapy could present a greater basis for pores and skin regeneration. This consists of elevated vascularity, extra localized appendage regeneration, and upregulation of follicle neogenesis-related expression. These enhancements are primarily as a result of inhibition of bottom-up patch restore and considerably lowered fibrosis, possible ensuing from the twin motion of inflammatory management and antibacterial, which normalizes collagen distribution and ECM association downstream. Lastly, with restraining fibroblast-to-myofibroblast transition and mitigating sustained profibrotic signaling, this modulation not solely facilitates orderly ECM transforming but additionally preserves a dermal structure that’s much less densely fibrotic and extra physiologically aligned. Such structural normalization is anticipated to revive applicable collagen fiber orientation and enhance tensile power, thereby reinforcing the useful integrity of regenerated pores and skin.
In vivo biocompatibility of PBPA hydrogel
After attaining promising therapeutic results, in vivo biotoxicity and compatibility have been evaluated. On the identical time, extreme deposition of silver is believed to result in in vivo toxicity, and so as to rule out such a threat, the next experiments have been carried out.
First the precise quantity of Ag utilized have been calculated as round 2.5 mg for every wound. This can be a localized utility, and the precise quantity coming into the systemic circulation could also be even decrease. Research have proven that mice consuming 110 mg of silver over one week exhibit no adversarial results [79, 80]. For people, the World Well being Group states {that a} lifetime consumption of 10 g of silver poses no well being dangers [80]. Medical examine additionally signifies that every day ingestion of fifty mg of silver doesn’t trigger any adversarial impact of silver toxicity [81]. Moreover, sure populations naturally have silver ranges as excessive as 100 g of their our bodies [80]. Due to this fact, utilizing 2.5 mg of silver to deal with a wound of roughly 0.5 cm² is unlikely to have vital adversarial results. To raised image, monitoring physique weight information all through the therapy interval confirmed a gradual enhance in all teams, with the PBPA2 + L group exhibiting the best weight acquire and the C group the least (Fig. S44). Hematological (Fig. S45) and biochemical (Fig. S46) analyses carried out on D14 indicated that the hydrogel parts in every group didn’t trigger any vital poisonous adjustments within the measured parameters. Notably, some indicators within the C group remained exterior the conventional vary. This systemic biocompatibility was additional confirmed by H&E staining of main organs, together with the center, liver, spleen, lungs, and kidneys (Fig. S47).
These outcomes show that PBPA2 hydrogel-guided antibacterial nanozyme therapy displays wonderful biocompatibility in vivo, with no detectable systemic toxicity. General, this therapeutic program provides exact, phase-specific management over wound therapeutic whereas addressing the structural limitations of typical therapies. Within the hemostatic section, PBPA2 + L quickly achieves bleeding management, debridement, thermal remedy, and biofilm disruption. Throughout irritation, ROS sensing triggers the managed launch of nanozymes, enabling environment friendly ROS and bacterial clearance, which shortens the inflammatory section and promotes transition to the proliferative section. Within the proliferative section, suppression of myofibroblast overactivation, mixed with the hydrogel’s low modulus and degradability, creates a good area of interest that accelerates re-epithelialization, fibroblast proliferation, and collagen deposition. Lastly, in transforming, these properties interrupt the stress–myofibroblast loop, enabling environment friendly ECM reorganization and appendage regeneration. In contrast to customary wound therapies that focus on just one side, antibiotics for bacterial clearance, antioxidants for ROS scavenging, or dressings that present passive protection, the hydrogel integrates these therapeutic dimensions right into a single spatiotemporally programmed system. By dynamically balancing ROS ranges, it resolves the dilemma of exploiting ROS for antibacterial motion whereas stopping oxidative tissue harm. Furthermore, by shifting therapeutic from a bottom-up, scar-filling trajectory to a peripheral-to-central regenerative mode, it establishes a complicated paradigm that permits true pores and skin regeneration somewhat than incomplete closure.
Nonetheless, this examine has a number of limitations that warrant additional investigation: (1) Whereas altered therapeutic orientation was related to elevated Ki67 expression and modified fibroblast exercise, the exact mechanisms-potentially involving collagen ratio shifts and vimentin-mediated remodeling-remain to be clarified. (2) The programmed therapy design, although efficient, nonetheless requires optimization, and handbook NIR irradiation compromises stability in comparison with autonomous platforms, underscoring the necessity for built-in and clinically protected supply programs. (3) Scalability and medical translation stay difficult, as large-area or deep wounds could demand uniform hydrogel utility and exact ROS modulation in heterogeneous environments. (4) Furthermore, though biofilm-infected wounds have been emphasised, diabetic wounds signify one other crucial however unaddressed continual mannequin, highlighting the necessity for broader validation earlier than medical translation.