Detection of nucleotide mutations
As a result of restricted availability of lengthy RNA samples with outlined mutations, we first validated RNA-SCAN utilizing MS2 RNA as a mannequin service scaffold (Supplementary Fig. 2) and artificial nanolatches carrying particular base adjustments (Fig. 2a). Particularly, we designed six nanolatch variants focusing on the MS2 RNA website, every carrying a definite sequence alteration: three with single-nucleotide substitutions at totally different positions (MHm1–MHm3), one with two substitutions (MHm4), one with an insertion (MHm5) and one with a deletion (MHm6). Every nanolatch was incubated individually with the service at a tenfold stoichiometric extra (Supplementary Fig. 3). The ensuing constructs had been analysed utilizing nanopore measurements.
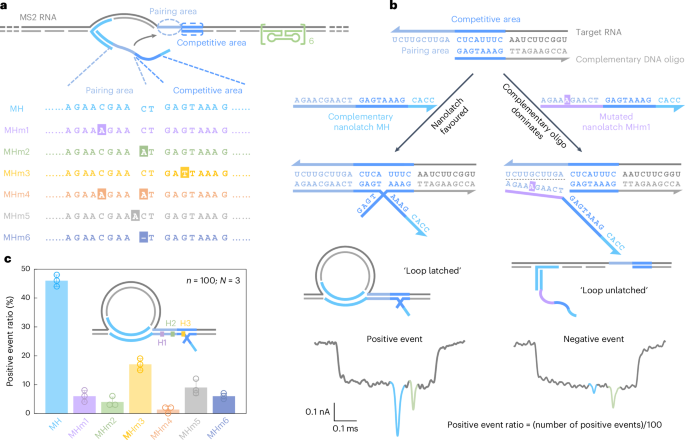
a, A sketch of the MS2 service design and sequence comparability between the complementary nanolatch (MH) and its six mutated variants (MHm1–MHm6), highlighting nucleotide adjustments in each pairing and aggressive areas that work together with the goal website on the MS2 service. b, The mechanism of the aggressive interplay between the MS2 RNA goal website and nanolatches/complementary DNA oligo, illustrated utilizing the absolutely complementary nanolatch MH and single-nucleotide mutated nanolatch MHm1. The consultant nanopore present traces exhibit distinct signatures comparable to the ‘loop latched’ (constructive occasion) and ‘loop unlatched’ (destructive occasion) states. The constructive occasion ratio is outlined because the variety of constructive occasions divided by the full variety of occasions per measurement (n = 100). c, An evaluation of constructive occasion ratios for nanolatches MH–MHm6. The bar charts current constructive occasion ratios from three unbiased measurements (N = 3) for every nanolatch. The information are proven because the imply ± commonplace deviation.
The sensitivity of RNA-SCAN to single-nucleotide variations stems from the finely tuned aggressive hybridization mechanism on the goal website (Fig. 2b). Particularly, every goal website is engineered with a 10-nt pairing space and an adjoining 8-nt aggressive space. When assembling the service, the aggressive space of the RNA scaffold is complemented by a DNA oligo, whereas the pairing space is deliberately left single-stranded to permit full nanolatch binding. This strategic design establishes a fragile competitors the place the goal RNA can transiently hybridize with both the nanolatch or the complementary DNA oligo. When the RNA sequence absolutely matches the nanolatch, their interplay is thermodynamically beneficial, yielding latched loop buildings, which manifest as attribute spike signatures in nanopore measurements (Fig. 2b, left). Nonetheless, even a single-nucleotide mismatch weakens the nanolatch-RNA binding affinity, tipping the steadiness in favour of the complementary oligo, which types a extra steady duplex with the RNA scaffold. This aggressive displacement of nanolatch will increase the chance of an unlatched state, characterised by the absence of the big present spike related to the latched configuration (Fig. 2b, proper). We classify translocation occasions as ‘constructive’ when the attribute spike is current and ‘destructive’ when it’s absent. A present threshold of 0.55 (Prolonged Knowledge Fig. 1) was utilized to facilitate classification. The constructive occasion ratio is outlined because the variety of constructive occasions out of 100 complete recorded occasions per nanopore measurement. Optimization of the pairing space and aggressive space is proven in Prolonged Knowledge Fig. 3.
Determine 2c exhibits the constructive occasion ratios calculated from the primary 100 linear nanopore occasions (n = 100) in every experiment, with error bars from three unbiased repeats (N = 3). This standardized quantification ensures dependable but environment friendly evaluation throughout circumstances (Supplementary Fig. 4). When the nanolatch sequence was absolutely complementary to the MS2 RNA goal website (MH), the constructive occasion ratio approached 50%. This degree displays the dynamic nature of loop formation and represents a steadiness optimized for single-nucleotide sensitivity. Within the presence of a single-nucleotide mismatch, the constructive occasion ratio constantly dropped, although the magnitude of discount relied on the mutation’s place. Mutations within the pairing space (MHm1 and MHm2) led to an approximate tenfold lower, with constructive occasion ratios falling to ~5%, whereas the change within the aggressive space (MHm3) resulted in a better common ratio of ~17%. That is most likely as a result of mutations within the aggressive space primarily have an effect on the competitors between the nanolatch and the complementary oligo with out disrupting the preliminary nanolatch-RNA pairing, thereby permitting loop formation to proceed extra readily. To make sure excessive sensitivity and specificity of RNA-SCAN, we strategically positioned mutated or chemically modified nucleotides throughout the pairing space in subsequent designs. Two mismatches (MHm4) additional suppressed loop formation, nearly approaching the detection restrict. Nucleotide insertion (MHm5) or deletion (MHm6) within the nanolatch additionally disrupted its interplay with the service, decreasing the constructive occasion ratio to under 10%. These outcomes display that RNA-SCAN is extremely delicate to refined sequence variations and may discriminate single-base variations by simply interpretable nanopore signatures (Prolonged Knowledge Fig. 4).
Detection of nucleotide modifications
Having demonstrated the power of RNA-SCAN to detect single-nucleotide mutations, we subsequent utilized this system to epigenetic nucleotide modifications, which play a vital position in lots of basic metabolic capabilities3,4.
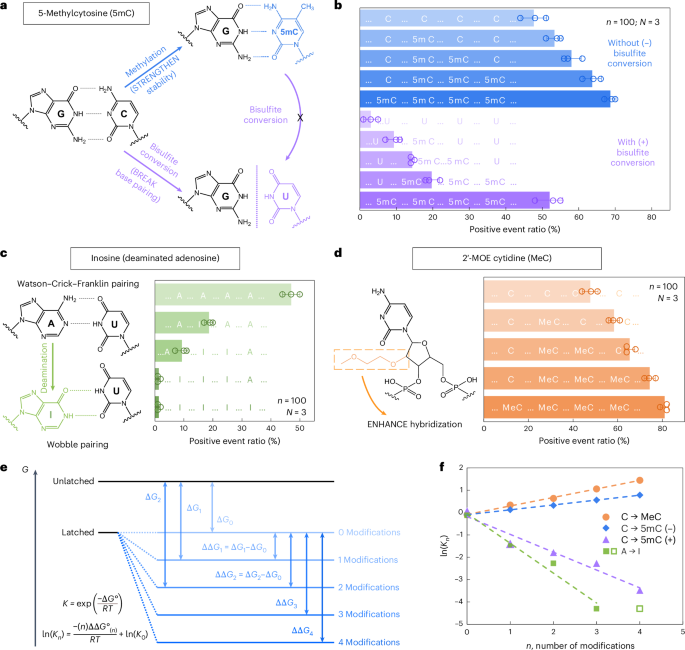
We first examined 5-methylcytosine (denoted as 5mC in DNA and m5C in RNA), a key epigenetic marker steadily noticed in most cancers21,22. To analyze its presence and abundance in nucleic acids, we evaluated the RNA-SCAN system from two complementary instructions. First, the covalent introduction of a methyl group to cytosine alters the van der Waals radii across the C5 place and modifies base stacking potential. Prior research have proven that such methylations can improve duplex stability by influencing thermodynamic properties23,24. Alternatively, bisulfite remedy can selectively deaminate unmethylated cytosines to uracil whereas leaving 5-methylcytosines intact25,26. This chemical conversion alters the base-pairing desire of cytosine from guanine to adenine, thereby disrupting hybridization patterns (Fig. 3a). Following these rules, we designed 5 nanolatch variants containing 0–4 5mC modifications and measured loop formation with and with out bisulfite conversion. The nanopore measurement outcomes are summarized in Fig. 3b. With out bisulfite conversion, rising the variety of 5mC modifications led to greater constructive occasion ratios, reaching ~69% for the four-modification nanolatch, which is sort of 1.5 occasions the ratio noticed with the unmodified management (~48%). With bisulfite conversion, the constructive occasion ratio for the four-methylated nanolatch remained comparatively excessive at ~52%, whereas nanolatches containing fewer 5mC modifications exhibited a considerable lower in constructive occasions. This sample means that bisulfite-induced deamination of unmethylated cytosines disrupted their pairing with guanines, destabilizing loop formation and resulting in unlatching.
a, A schematic depicting how 5mC modification influences C/G base pairing earlier than and after bisulfite conversion. The MS2 service design is equivalent to that in Fig. 2. b, The nanopore measurement outcomes of MS2 carriers incubated with nanolatches containing 0–4 5mC modifications, with (+) and with out (−) bisulfite remedy. c, A schematic of how inosine modification impacts A/U base pairing, together with nanopore measurement outcomes for nanolatches containing 0–4 inosines within the sequence. d, A schematic of MeC modification and nanopore measurement outcomes for nanolatches possessing 0–4 MeCs. The information in b–d are proven because the imply ± commonplace deviation from three unbiased measurements. e, A theoretical mannequin of the nanolatch system for detecting modifications. The presence of modifications can both strengthen or weaken the steadiness of the duplex between the nanolatch and the RNA scaffold, altering the power distinction (Delta G) between ‘latched’ and ‘unlatched’ power ranges. R is the perfect fuel fixed, T is absolutely the temperature at which the response happens, ({Okay}_{n}) is the equilibrium fixed of loop latching, and (n) is the variety of modifications. f, The linear becoming outcomes of (mathrm{ln}({Okay}_{n})) towards (n) for every sort of modifications studied on this work. 5mC (+) and 5mC (−) check with cytosine methylations with (+) and with out (−) bisulfite remedy, respectively. The information level for 4 inosines (open sq.) was excluded from becoming, because the constructive occasion ratio had already dropped to almost 0% with three inosines.
We subsequent examined the power of RNA-SCAN to detect inosine, a product of adenosine deamination that’s related to RNA enhancing and post-transcriptional gene regulation27,28. Chemically, this amino-to-keto conversion alters the bottom’s hydrogen bonding properties, remodeling adenosine from a hydrogen bond donor into an acceptor. Because of this, canonical Watson–Crick–Franklin base pairing with uridine is changed by two weaker, non-canonical wobble hydrogen bonds29,30 (Fig. 3c, left). This transformation destabilizes the RNA/DNA duplex and alters loop formation throughout the service. To guage this impact, we designed 5 nanolatches containing 0–4 inosine substitutions. Nanopore measurements (Fig. 3c, proper) confirmed that even a single inosine substitution diminished the constructive occasion ratio from ~47% to ~19%. With three inosines current, loop formation was nearly fully disrupted, and the sign dropped to close zero. These outcomes affirm that RNA-SCAN can sensitively detect the structural penalties of inosine incorporation.
To evaluate RNA-SCAN’s capacity to detect sugar modifications, we included 2′-O-methoxyethyl (2′-MOE) teams into cytidines (2′-MOE cytidine, MeC), that are recognized to reinforce duplex stability in antisense oligo therapeutics31,32 (Fig. 3d, left). Substituting 5mC with MeC in nanolatches led to even better constructive alerts (Fig. 3d, proper): 4 MeC modifications yielded ~81% constructive occasions, in contrast with ~69% with 5mC and ~48% with unmodified sequences. Even a single MeC elevated sign by over 10%, highlighting this modification’s robust stabilizing impact on base pairing.
To interpret these findings, we modelled loop formation as a two-state power system: latched versus unlatched (Fig. 3e). Modifications shift the power of the latched state, altering its equilibrium. Based mostly on the nearest-neighbour mannequin33,34, which assumes additive energetic contributions from adjoining base pairs, we noticed robust linear correlations between the variety of modifications and the equilibrium fixed (Fig. 3f). These tendencies aligned with our experimental outcomes and assist the thermodynamic foundation for modification detection. Additional particulars can be found in Supplementary Notice 2.
Discrimination of E. coli and S. Typhi by RNA-SCAN
To guage the efficiency of RNA-SCAN on naturally occurring lengthy RNA, we utilized it to distinguish between Escherichia coli and Salmonella enterica serovar Typhi (S. Typhi), two carefully associated Gram-negative micro organism of medical relevance. Whereas most E. coli strains are innocent intestine commensals, some pathogenic variants trigger extreme gastrointestinal sickness35,36. S. Typhi, a human-specific pathogen, is accountable for enteric (typhoid) fever and presents distinct antimicrobial resistance profiles in contrast with E. coli37,38.
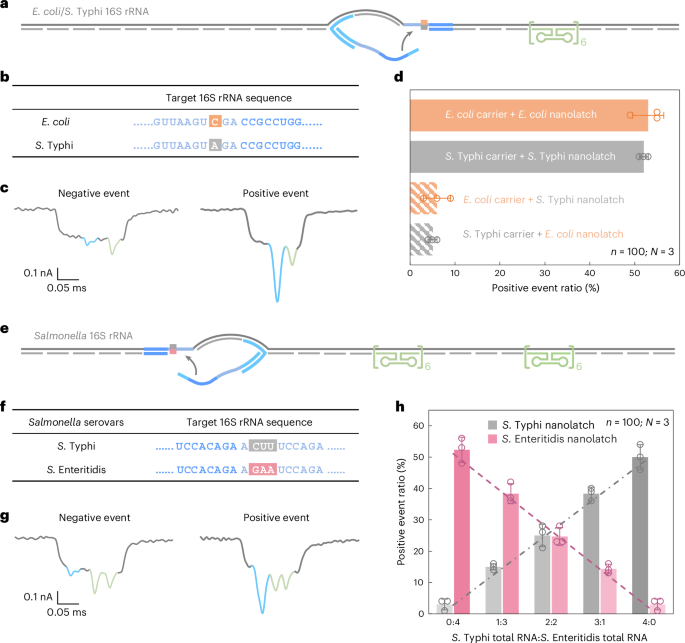
To allow species-level discrimination, we centered on 16S ribosomal RNA (rRNA), a extremely conserved however diagnostically informative bacterial identification marker. Sequence alignment throughout all 14 operons from each species revealed >99% intraspecies similarity and 97–98% interspecies similarity (Supplementary Fig. 5). Inside this context, we recognized an 18-nt area containing a constant single-nucleotide distinction between E. coli and S. Typhi 16S rRNA and chosen it because the goal for RNA-SCAN detection (Fig. 4a,b). Separate oligo swimming pools had been designed to assemble RNA carriers from the full 16S rRNA of every species, whereas nanolatches had been tailor-made to match the species-specific variant of the goal sequence. Every service was incubated with both a matched or mismatched nanolatch, creating 4 experimental mixtures.
a, A schematic of the E. coli/S. Typhi 16S rRNA service design with a reference construction positioned on one facet of the service and a goal website situated on the centre. b, The sequences of E. coli and S. Typhi 16S rRNA on the goal website, highlighting a single-nucleotide variation. c, Consultant nanopore present traces of the E. coli/S. Typhi 16S rRNA service. d, The evaluation of the constructive occasion ratio for the 4 mixtures of E. coli/S. Typhi service with E. coli/S. Typhi nanolatch. The service focus was set at 0.3 nM at the beginning of nanopore measurements and the nanolatch focus at 3 nM for each E. coli and S. Typhi. e, A schematic of the Salmonella 16S rRNA service design with two reference buildings positioned on one facet and a goal website on the opposite facet. f, The sequences of S. Typhi and S. Enteritidis 16S rRNA on the goal website, highlighting three steady totally different bases. g, Consultant nanopore present traces of the Salmonella 16S rRNA service. h, An evaluation of the constructive occasion ratio for complete RNA mixtures of S. Typhi and S. Enteritidis at outlined enter ratios, utilizing both the S. Typhi or S. Enteritidis nanolatch. The mixed focus of S. Typhi and S. Enteritidis 16S rRNA carriers was maintained at 0.3 nM in all nanopore measurements, with a continuing nanolatch focus of three nM. The information in d and h are proven as imply ± commonplace deviation from three unbiased measurements.
Nanopore measurements had been performed utilizing complete RNA straight extracted from E. coli and S. Typhi cultures, with out extra purification or enrichment. In matched mixtures—E. coli service with E. coli nanolatch or S. Typhi service with S. Typhi nanolatch—the constructive occasion ratio exceeded 50%, indicating profitable loop formation (Fig. 4d). Against this, the mismatched mixtures yielded constructive occasion ratios of round 5%, demonstrating a transparent lack of nanolatch binding. This almost tenfold distinction in sign underscores the excessive sensitivity and specificity of RNA-SCAN in distinguishing single-nucleotide variations inside lengthy, native RNA molecules (Prolonged Knowledge Fig. 5). Importantly, the assay requires no enzymatic processing, amplification or fragmentation and works straight with complete bacterial RNA, making it extremely appropriate for sensible functions akin to microbial identification and resistance profiling.
Quantification of Salmonella 16S rRNA variants
Whereas the excessive sequence similarity of 16S rRNA between E. coli and S. Typhi already poses a problem for standard assays, totally different serovars of Salmonella enterica, akin to S. Typhi and S. Enteritidis39, are much more tough to differentiate, with 16S rRNA sequence identities exceeding 99.5% overlap (Supplementary Fig. 6).
To deal with this, we designed a shared oligo pool that binds conserved areas in each S. Typhi and S. Enteritidis 16S rRNA, utilizing consensus nucleotides at polymorphic websites. The service design included two reference buildings on one facet to differentiate this assemble from earlier E. coli/S. Typhi carriers (Fig. 4e and Prolonged Knowledge Fig. 6) and a nanolatch binding website on the opposite facet the place three consecutive nucleotides differ between the 2 serovars (Fig. 4f). To imitate medical circumstances the place RNA from a number of bacterial species could coexist, we combined complete RNA extracted from S. Typhi and S. Enteritidis in outlined ratios. After hybridizing the combination with the oligo pool to generate carriers for each serovars, every combination was cut up and incubated with both a S. Typhi-specific or S. Enteritidis-specific nanolatch.
The nanopore measurements confirmed that the constructive occasion ratio elevated linearly with the proportion of every goal RNA within the combination when probed with its matching nanolatch (Fig. 4h). This linear relationship confirms that RNA-SCAN cannot solely distinguish between extremely related sequences but additionally quantify their relative abundance in complicated RNA backgrounds. The small error bars and constant matches additional point out that different minor sequence variations or intraspecies polymorphisms didn’t intrude with detection. These outcomes spotlight the potential of RNA-SCAN for multiplexed pathogen quantification in combined samples, with single-nucleotide decision and with out requiring prior pattern purification or amplification.
m5C detection in 16S rRNA from E. coli and A. baumannii
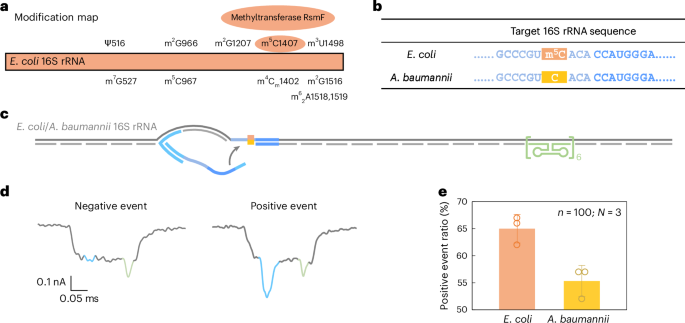
Past sequence variation, we additional evaluated the power of RNA-SCAN capacity to detect base modifications in endogenous lengthy RNA. In E. coli, rRNAs comprise a minimum of 24 recognized methylation websites important for ribosomal perform40. Amongst these, three are m5C modifications situated at positions C967 and C1407 in 16S rRNA (Fig. 5a) and at C1962 in 23S rRNA. Notably, methylation at C1407 is extremely conserved in E. coli and has been implicated in translation constancy40,41. Against this, Acinetobacter baumannii, a typical reason for healthcare-associated opportunistic infections, fully lacks the methyltransferase gene accountable for C1407 methylation42,43. This makes E. coli and A. baumannii perfect comparative fashions for learning RNA methylation at this place.
a, A modification map of E. coli 16S rRNA, highlighting the important position of the methyltransferase RsmF in catalysing methylation at cytosine 1407. b, The sequences of E. coli and A. baumannii 16S rRNA on the goal website. The cytosine at place 1407 is methylated in E. coli 16S rRNA however stays unmethylated in A. baumannii 16S rRNA. c, A schematic of the E. coli/A. baumannii 16S rRNA service design, that includes a reference construction on one facet of the service and the goal website on the reverse finish. d, Consultant nanopore present traces of the E. coli/A. baumannii 16S rRNA service. e, The evaluation of the constructive occasion ratio for E. coli and A. baumannii 16S rRNA carriers utilizing the identical nanolatch. The service focus was maintained at 0.3 nM and the nanolatch focus at 3 nM. The upper constructive occasion ratio noticed for E. coli will be attributed to the stabilizing impact of the m5C modification on the goal website. The information are proven as imply ± commonplace deviation from three unbiased measurements.
Given the substantial sequence divergence of over 15% between their 16S rRNA operons (Supplementary Fig. 7), we designed species-specific oligo swimming pools for assembling RNA carriers, whereas utilizing a typical nanolatch focusing on the C1407 area. The ensuing service construction included the C1407 goal website on one facet and a reference construction on the opposite (Fig. 5b,c). The entire RNA extracted straight from bacterial cells was used with out additional processing.
Nanopore measurements confirmed a statistically important distinction in loop formation between species. E. coli produced a constructive occasion ratio of 65% ± 2%, in contrast with 55% ± 2% for A. baumannii (Fig. 5e and Prolonged Knowledge Fig. 7), with a t-test yielding P = 0.01289. These outcomes are in line with our earlier MS2-based experiments, supporting the interpretation that m5C1407 enhances nanolatch binding by stabilizing C/G base pairing. Apparently, each species exhibited greater sign ranges than unmodified MS2 carriers. To analyze this, we carried out bisulfite sequencing on the 16S rRNA goal area. The outcomes (Prolonged Knowledge Fig. 8) counsel that one other close by modification, m4Cm1402 (a 2′-O-methylated and N4-methylated cytosine present in each species), is partly immune to bisulfite conversion and should equally strengthen base pairing.
Collectively, these findings display the potential of RNA-SCAN to detect single-base RNA modifications in native, unamplified samples. Furthermore, the commentary of enhanced alerts from close by modified nucleotides means that RNA-SCAN may additionally function a instrument for figuring out beforehand unannotated modifications in functionally essential RNA areas.