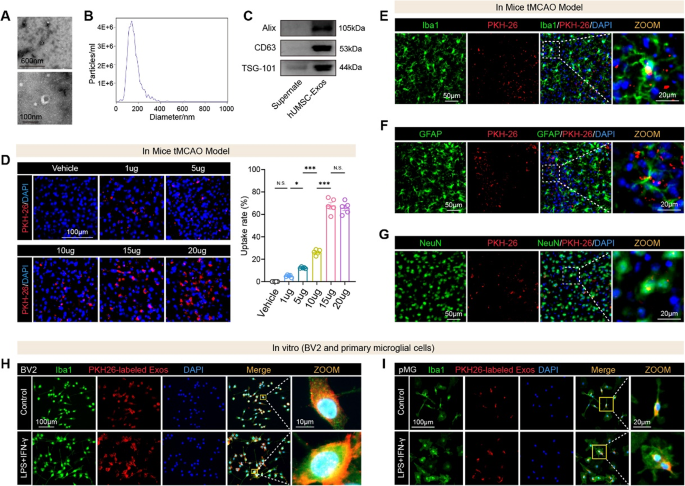
Intranasal hUMSC-Exos goal a number of mind cell sorts and accumulate within the ischemic penumbra
We first remoted and characterised hUMSC-Exos to make sure their purity and performance. Transmission electron microscopy (TEM) revealed their attribute cup-shaped morphology (Fig. 1A). Nanoparticle monitoring evaluation (NTA) confirmed a uniform dimension distribution with a median diameter of 144.9 nm (Fig. 1B; Fig. S1). Western blotting detected classical exosomal markers, together with Alix, CD63, and TSG101, validating profitable isolation (Fig. 1C). To evaluate in vivo biodistribution, we labeled hUMSC-Exos with PKH26 and administered them intranasally instantly after tMCAO. Fluorescence imaging at 12 h revealed substantial accumulation in each the ischemic and contralateral hemispheres, with considerably greater depth within the ischemic areas in comparison with PBS-treated controls (Fig. S2). We subsequent optimized the dosing routine. Mice obtained escalating doses of PKH26-labeled hUMSC-Exos instantly post-tMCAO. Quantitative evaluation of mind sections 12 h later confirmed a dose-dependent enhance in exosome uptake throughout the ischemic penumbra, peaking at 15 µg (Fig. 1D). Doses above 15 µg didn’t additional improve uptake, establishing 15 µg because the optimum therapeutic dose. To determine the mobile targets, we carried out immunofluorescence staining. PKH26-labeled hUMSC-Exos colocalized with Iba1⁺ microglia (Fig. 1E), GFAP⁺ astrocytes (Fig. 1F), and NeuN⁺ neurons (Fig. 1G) within the ischemic penumbra. To additional validate microglial uptake beneath inflammatory circumstances, we carried out in vitro assays utilizing BV2 and first microglial (pMG) cultures stimulated with LPS and IFN-γ. Activated BV2 cells (Fig. 1H) and pMG (Fig. 1I) exhibited markedly enhanced uptake of PKH26-labeled exosomes in comparison with non-activated controls.
Collectively, these findings reveal that intranasally delivered hUMSC-Exos successfully penetrate the mind, preferentially accumulate in ischemic areas, and are internalized by key neural cell sorts, positioning them as promising therapeutic vectors for post-stroke neuroprotection.
Characterization, dose optimization, and mobile uptake of hUMSC-derived exosomes (hUMSC-Exos) in vivo and in vitro. A Transmission electron microscopy photographs reveal the attribute cup-shaped morphology of remoted hUMSC-Exos. B Nanoparticle monitoring evaluation (NTA) reveals a dimension distribution with a peak diameter in keeping with exosomal profiles. C Western blot evaluation confirms the enrichment of canonical exosomal markers (Alix, CD63, and TSG101) in hUMSC-Exos in comparison with the supernatant. D Consultant immunofluorescence photographs of mind sections from mice subjected to tMCAO and handled with escalating doses of intranasally delivered PKH26-labeled hUMSC-Exos. Quantification reveals considerably elevated uptake at 15 µg in comparison with decrease doses and automobile. n = 5 mice per group. NS = not important, *p < 0.05, ***p < 0.001. E-G Immunofluorescence imaging of mind sections from tMCAO mice 12 h after intranasal hUMSC-Exo administration. Colocalization of PKH26⁺ indicators with Iba1⁺ microglia, GFAP⁺ astrocytes, and NeuN⁺ neurons verify widespread mobile uptake throughout the ischemic penumbra. H In vitro uptake of PKH26-labeled hUMSC-Exos by BV2 microglia beneath management or LPS + IFN-γ-stimulated circumstances. I Main microglia (pMG) additionally exhibit enhanced uptake of hUMSC-Exos following inflammatory stimulation, as indicated by colocalization of PKH26 sign with Iba1⁺ cells
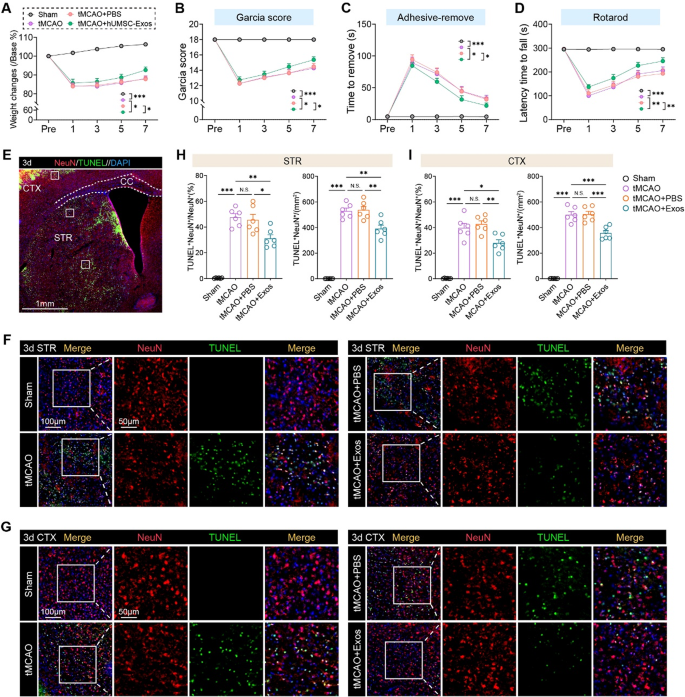
hUMSC-Exos improve useful restoration and attenuate neuronal apoptosis after cerebral ischemia
To research the therapeutic efficacy of hUMSC-Exos in cerebral ischemia, we employed a tMCAO mouse mannequin adopted by intranasal exosome administration. hUMSC-Exo therapy considerably accelerated post-stroke restoration. In comparison with the tMCAO and PBS-treated teams, exosome-treated mice confirmed extra fast weight acquire (Fig. 2 A), improved composite Garcia scores (Fig. 2B), and enhanced positive motor restoration as indicated by lowered adhesive removing occasions (Fig. 2 C). Moreover, motor coordination and endurance have been preserved within the hUMSC-Exo group, as proven by extended latency on the rotarod check (Fig. 2D).
hUMSC-Exos promote neurofunctional restoration and cut back neuronal apoptosis following cerebral ischemia. A–D Neurological restoration was assessed in mice subjected to tMCAO and handled with PBS or hUMSC-Exos by way of intranasal administration. hUMSC-Exo-treated mice exhibited improved restoration as evidenced by enhanced weight acquire (A), elevated Garcia rating (B), lowered adhesive removing latency (C), and extended rotarod retention time (D) post-stroke. N = 11 per group. Two-way ANOVA check. E Consultant overview of mind Sect. 3 days post-tMCAO, highlighting cortex (CTX) and striatum (STR) areas used for TUNEL/NeuN evaluation. F-G Consultant immunofluorescence photographs displaying TUNEL (inexperienced), NeuN (purple), and DAPI (blue) staining in STR (F) and CTX (G). Apoptotic neurons (TUNEL⁺NeuN⁺) have been markedly lowered in hUMSC-Exo-treated animals. H-I Quantification of apoptotic neurons within the STR (H) and CTX (I). N = 6 per group. Scale bars as indicated. One-way ANOVA with Tukey’s submit hoc check. Information are offered as imply ± SEM. *p < 0.05, **p < 0.01, and ***p < 0.001. “ns” signifies no important distinction
To guage neuronal survival, we carried out TUNEL and NeuN co-staining within the ischemic hemisphere at 3 days post-stroke. Apoptotic neurons (TUNEL⁺NeuN⁺) have been considerably lowered in each the striatum and cortex of hUMSC-Exo-treated animals relative to untreated and PBS controls (Fig. 2E–I). These outcomes reveal that hUMSC-Exos exert neuroprotective results by mitigating neuronal apoptosis in ischemic areas, which probably underlies the noticed enchancment in neurofunctional outcomes. These findings point out hUMSC-Exos exert neuroprotection by mitigating neuronal apoptosis, underpinning improved neurofunctional outcomes and supporting their function as a non-invasive, cell-free remedy.
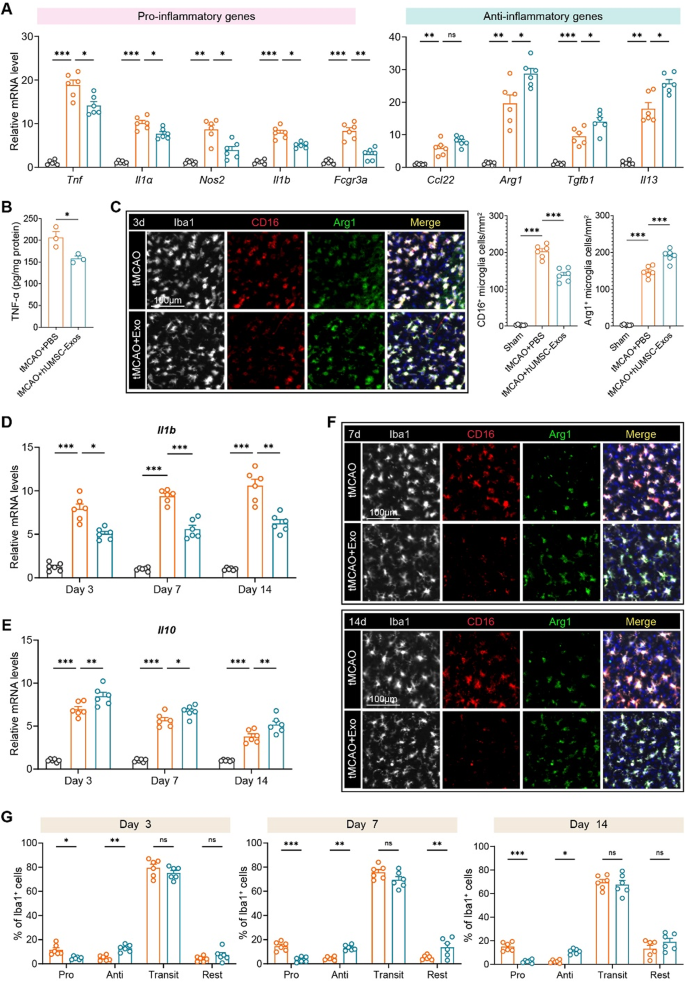
hUMSC-derived exosomes shift microglial polarization and suppress irritation post-stroke
Peri-infarct cytokine expression and microglial polarization have been analyzed throughout post-tMCAO time factors to evaluate neuroinflammation modulation by hUMSC-Exos. At day 3, qRT-PCR revealed a marked suppression of pro-inflammatory genes together with Tnf, Il1a, Nos2, Il1b, and Fcgr3a, together with important upregulation of anti-inflammatory genes reminiscent of Arg1, Tgfb1, and Il13 within the hUMSC-Exo-treated group (Fig. 3A). Correspondingly, TNF-α protein ranges have been considerably decreased (Fig. 3B). Immunostaining at day 3 additional confirmed that exosome administration lowered the proportion of CD16⁺ (pro-inflammatory) microglia and elevated Arg1⁺ (anti-inflammatory) cells amongst Iba1⁺ microglia (Fig. 3C). Longitudinal evaluation confirmed sustained downregulation of Il1b and elevation of Il10 mRNA throughout days 3, 7, and 14 (Fig. 3D–E). Confocal imaging at later time factors (days 7 and 14) demonstrated that hUMSC-Exo therapy continued to advertise a shift in microglial phenotype away from CD16⁺ and towards Arg1⁺ expression (Fig. 3F).
To quantitatively outline microglial heterogeneity, we categorized Iba1⁺ cells into 4 phenotypes: pro-inflammatory (CD16⁺Arg1⁻), anti-inflammatory (CD16⁻Arg1⁺), transitional (CD16⁺Arg1⁺), and resting (CD16⁻Arg1⁻). hUMSC-Exo considerably lowered pro-inflammatory microglia and elevated anti-inflammatory populations in any respect time factors, with out altering the transitional populations (Fig. 3G). These information reveal hUMSC-Exos reprogram microglia towards neuroprotective phenotypes and attenuate acute/sustained irritation.
hUMSC-derived exosomes modulate microglial polarization and suppress neuroinflammation after ischemic stroke. A Quantitative RT-PCR evaluation of pro-inflammatory (Tnf, Il1a, Nos2, Il1b, Fcgr3a) and anti inflammatory (Arg1, Tgfb1, Il13) genes in peri-infarct tissue at day 3 post-tMCAO. B ELISA quantification of TNF-α protein ranges within the peri-infarct cortex confirmed a big discount within the hUMSC-Exo group at day 3. C Immunofluorescence staining of microglial markers CD16 (purple), Arg1 (inexperienced), and Iba1 (white) at day 3 post-tMCAO. hUMSC-Exo therapy decreased CD16⁺ and elevated Arg1⁺ microglia (quantified at proper). D–E Longitudinal qPCR evaluation of Il1b (D) and Il10 (E) mRNA expression in peri-infarct tissue at days 3, 7, and 14. hUMSC-Exo lowered Il1b and elevated Il10 expression in any respect time factors. F Consultant confocal photographs of Iba1⁺ microglia co-stained with CD16 and Arg1 at days 7 and 14. G Quantification of microglial subpopulations (Professional: CD16⁺Arg1⁻, Anti: CD16⁻Arg1⁺, Transit: CD16⁺Arg1⁺, Relaxation: CD16⁻Arg1⁻) amongst Iba1⁺ cells at days 3, 7, and 14 post-tMCAO. N = 6 per group. Scale bars as indicated. One-way ANOVA with Tukey’s submit hoc check. Information are offered as imply ± SEM. *p < 0.05, **p < 0.01, and ***p < 0.001. “ns” signifies no important distinction
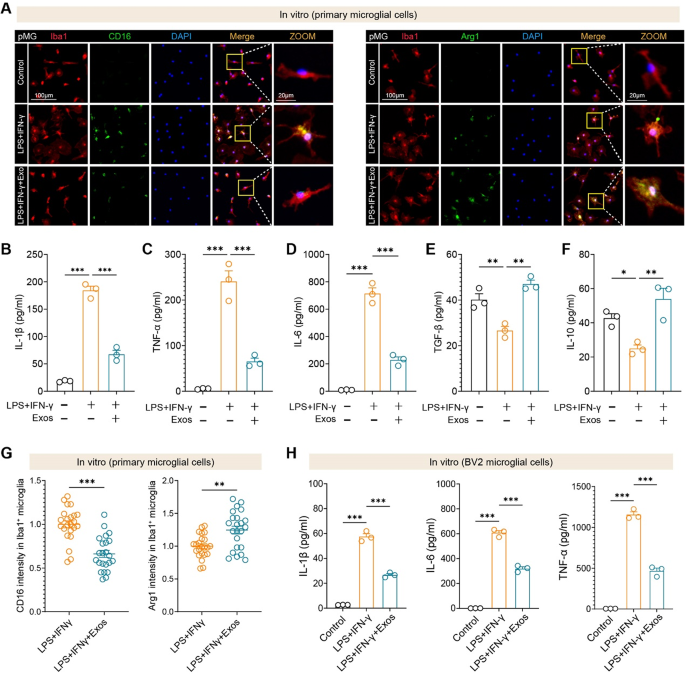
hUMSC-Exos attenuate microglial inflammatory activation and promote anti-inflammatory responses in vitro
To immediately assess the immunomodulatory results of hUMSC-Exos on microglia, we handled each main microglial cells (pMG) and BV2 cells with LPS + IFN-γ to induce an inflammatory response in vitro. Immunofluorescence evaluation confirmed that hUMSC-Exos considerably lowered CD16 expression and enhanced Arg1 expression in Iba1⁺ main microglia. Quantitative picture evaluation additional confirmed the discount in CD16 depth and the elevation of Arg1 depth in microglia uncovered to hUMSC-Exos (Fig. 4A, G). In line with these phenotypic adjustments, ELISA revealed that exosome therapy markedly suppressed the secretion of key pro-inflammatory cytokines, together with IL-1β, TNF-α, and IL-6 (Fig. 4B–D), whereas selling the manufacturing of anti-inflammatory mediators reminiscent of TGF-β and IL-10 (Fig. 4E–F). Importantly, comparable anti-inflammatory results have been noticed within the BV2 microglial cell line, the place hUMSC-Exos suppressed IL-1β, IL-6, and TNF-α ranges (Fig. 4H).
These findings reveal that hUMSC-derived exosomes successfully reprogram each main and immortalized microglia towards an anti-inflammatory phenotype and attenuate cytokine-driven neuroinflammation in vitro.
hUMSC-derived exosomes suppress pro-inflammatory responses and promote anti-inflammatory phenotypes in microglia in vitro. A Consultant immunofluorescence photographs of main microglial cells (pMG) handled with LPS + IFN-γ, with or with out hUMSC-derived exosomes (Exos), displaying expression of CD16 (left) and Arg1 (proper) in Iba1⁺ microglia. Scale bars as indicated. B–D ELISA quantification of pro-inflammatory cytokines IL-1β (B), TNF-α (C), and IL-6 (D) in supernatants of main microglia. Exosome therapy considerably lowered cytokine secretion induced by LPS + IFN-γ. E–F Anti-inflammatory cytokines TGF-β (E) and IL-10 (F) have been elevated following hUMSC-Exo therapy in main microglia. G Quantification of CD16 and Arg1 fluorescence depth in Iba1⁺ cells reveals lowered CD16 and elevated Arg1 ranges upon exosome therapy. N = 23 cells per group. H Related suppression of IL-1β, IL-6, and TNF-α was noticed in BV2 microglial cells handled with LPS + IFN-γ and hUMSC-Exos. N = 3 per group. Information are offered as imply ± SEM. *p < 0.05, **p < 0.01, and ***p < 0.001 by one-way ANOVA or t-test as applicable
hUMSC-Exos modulate microglial activation and inflammatory pathways to advertise neuroprotection post-stroke
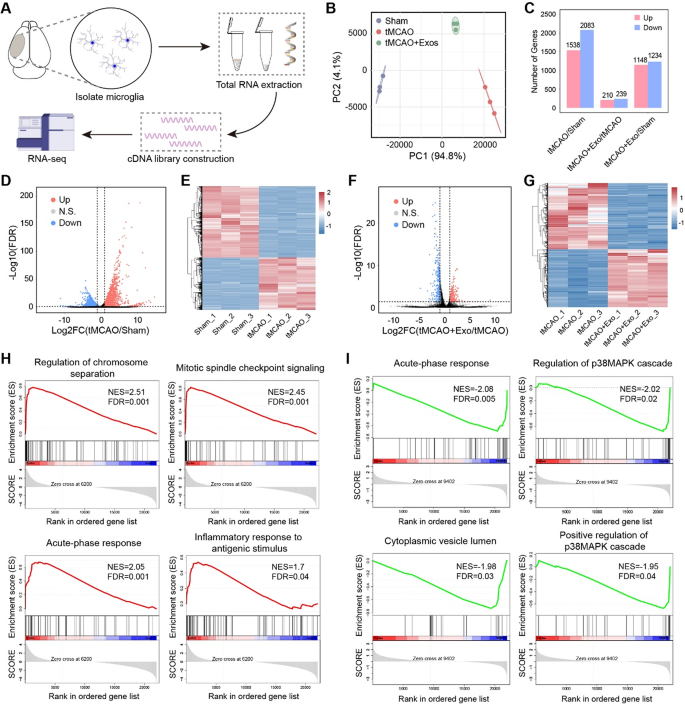
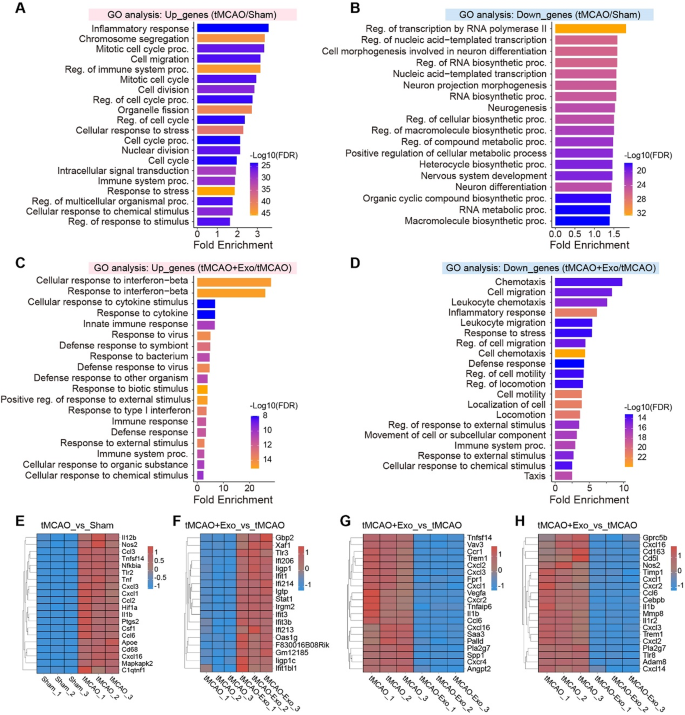
To research the molecular mechanisms underlying the anti-inflammatory results of hUMSC-Exos, RNA-seq was carried out on remoted microglia from Sham, tMCAO, and tMCAO + Exo teams (Fig. 5A). qRT-PCR confirmed the enrichment of microglia-specific marker genes, validating the purity of remoted microglia (Fig. S3). The RNA-seq information demonstrated prime quality and consistency throughout experimental teams (Fig. S4), with PCA evaluation displaying distinct transcriptional profiles amongst teams (Fig. 5B). Differential gene expression evaluation revealed that tMCAO induced important transcriptional adjustments, with 1538 upregulated and 2083 downregulated genes in comparison with Sham (Fig. 5C–E). In distinction, hUMSC-Exos therapy mitigated these results, with 210 genes upregulated and 239 downregulated in comparison with tMCAO (Fig. 5F–G). GSEA enrichment evaluation revealed that tMCAO activated inflammatory and proliferative pathways, reminiscent of acute-phase response and mitotic spindle checkpoint signaling (Fig. 5H). Nonetheless, hUMSC-Exos therapy suppressed these pathways, together with the p38MAPK cascade and cytoplasmic vesicle lumen, indicating lowered microglial activation and proliferation (Fig. 5I). GO enrichment evaluation of DEGs confirmed that tMCAO microglia upregulated pathways associated to irritation, cell division, and migration (Fig. 6A–B). In distinction, post-Exo therapy, these pathways have been downregulated, whereas immune-regulatory pathways, such because the interferon-β response, have been upregulated (Fig. 6C–D). Notably, inflammatory genes (e.g., Il1b, Tnf, Nos2) have been suppressed following Exo therapy, whereas immune-modulatory genes (e.g., Ifit3, Ifi206) have been upregulated (Fig. 6E–H).
These findings counsel that hUMSC-Exos promote a shift in microglial polarization towards an anti-inflammatory phenotype, lowering microglial activation, migration, and proliferation, whereas enhancing immune-regulatory responses. This molecular reprogramming contributes to the noticed neuroprotective results post-stroke.
hUMSC-Exos alter the transcriptional profile of microglia by lowering irritation and proliferation-related pathways submit stroke. A Experimental workflow of RNA-seq evaluation, together with microglia isolation from three teams (Sham, tMCAO, and tMCAO + Exo), RNA extraction, and cDNA library building. B PCA plot reveals clear clustering inside every group and distinct separation between teams, indicating important transcriptional variations. C Comparability of differentially expressed genes (DEGs) reveals 1538 upregulated and 2083 downregulated genes in tMCAO vs. Sham; 210 upregulated and 239 downregulated genes in tMCAO + Exo vs. tMCAO. D–E Volcano plot and heatmap of tMCAO vs. Sham present the distribution and expression of DEGs between the teams. F–G Volcano plot and heatmap of tMCAO + Exo vs. tMCAO spotlight the distribution and expression of DEGs between these two teams. H tMCAO vs. Sham reveals upregulation of pathways related to irritation and microglial proliferation. I tMCAO + Exo vs. tMCAO reveals downregulation of inflammatory pathways reminiscent of acute-phase response, and p38MAPK signaling
GO enrichment and consultant gene expression evaluation of microglia after tMCAO and hUMSC-Exos therapy. A Upregulated genes in tMCAO vs. Sham are enriched in pathways associated to inflammatory response, cell division, chromosome segregation, and cell migration, reflecting sturdy microglial activation and proliferation. B Downregulated genes are related to transcription regulation, RNA biosynthesis, neurogenesis, and neuron differentiation. C Upregulated genes in Exo-treated microglia are enriched in immune-regulatory pathways, together with interferon-β response, cytokine stimulus response, and innate immune response, suggesting a shift in the direction of an immunomodulatory phenotype. D Downregulated genes in Exo-treated microglia are related to chemotaxis, cell migration, and inflammatory response, indicating lowered pro-inflammatory exercise and diminished microglial overactivation. E Heatmap of consultant genes within the inflammatory response pathway upregulated in tMCAO vs. Sham. F Heatmap of interferon-β response genes upregulated in tMCAO + Exo vs. tMCAO. G-H Heatmaps of inflammatory response and chemotaxis genes downregulated in tMCAO + Exo vs. tMCAO
hUMSC-Exos goal TREM1 to suppress microglial inflammatory activation, migration, and proliferation
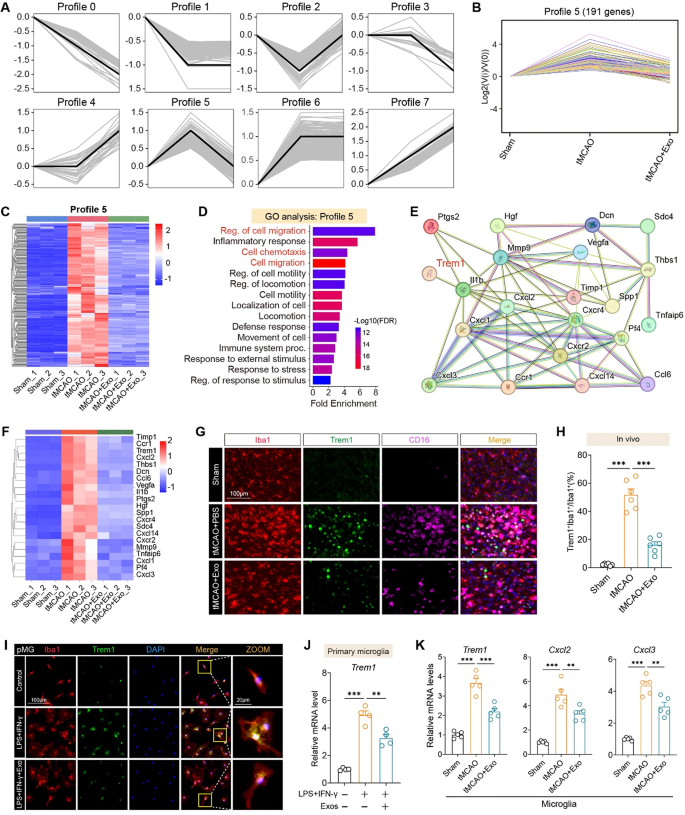
Pattern clustering revealed eight distinct expression profiles, amongst which Profile 5 contained 191 genes displaying a “low–excessive–low” development—upregulated after stroke and downregulated by exosome therapy (Fig. 7A–B). Heatmap evaluation confirmed constant modulation of those genes throughout particular person replicates (Fig. 7C). GO enrichment of Profile 5 genes highlighted processes associated to cell migration, chemotaxis, and inflammatory response (Fig. 7D). A protein–protein interplay (PPI) community recognized TREM1 as a central hub linking a number of chemokines and inflammatory mediators reminiscent of Cxcl2, Cxcl3, Ccl6, and Vegfa (Fig. 7E–F). Immunofluorescence in mind sections confirmed that TREM1 expression was elevated in Iba1⁺ microglia after tMCAO and considerably lowered following Exo therapy (Fig. 7G–H). In vitro, main microglia stimulated with LPS and IFN-γ exhibited elevated Trem1 expression, which was markedly lowered upon hUMSC-Exo administration, as proven by immunofluorescence (Fig. 7I; Fig. S5A) and qRT-PCR (Fig. 7J). In vivo, hUMSC-Exos suppressed the expression of Trem1, Cxcl2, and Cxcl3 in FACS-sorted microglia from tMCAO mice (Fig. 7Ok), and in addition downregulated Ccl6, Vegfa, and Mmp9 mRNA ranges (Fig. S5B). Furthermore, transcript evaluation of entire cortical tissue revealed that Exo therapy considerably lowered ischemia-induced upregulation of Trem1, Cxcl2, Cxcl3, Ccl6, and Mmp9 (Fig. S5C). Collectively, these information reveal that hUMSC-Exos attenuate neuroinflammation by suppressing pro-inflammatory gene expression in activated microglia each in vitro and in vivo after ischemic stroke.
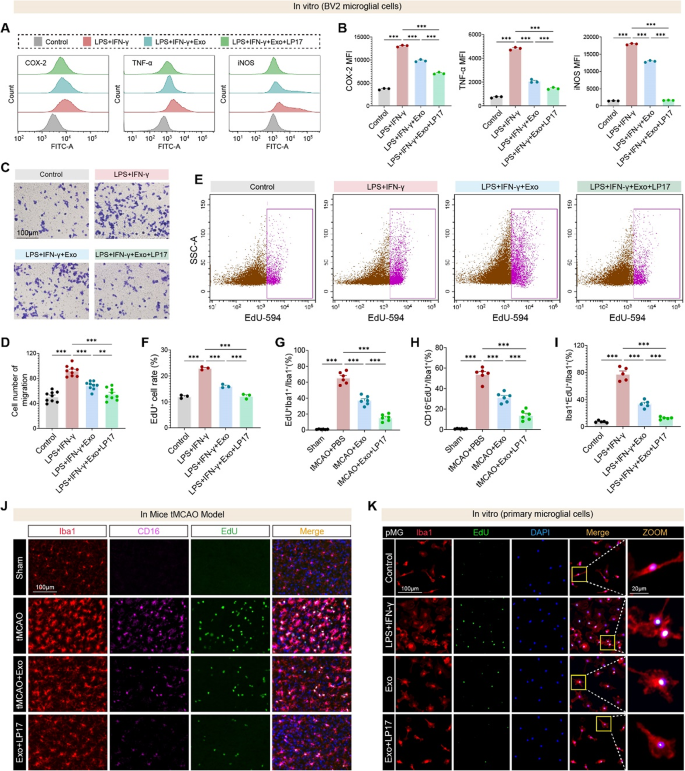
To check the useful relevance of TREM1 in microglial activation, we evaluated its function in inflammatory signaling, migration, and proliferation utilizing each ex vivo and in vivo approaches. Circulate cytometry confirmed that LPS + IFN-γ stimulation elevated expression of COX-2, TNF-α, and iNOS, which have been suppressed by hUMSC-Exos, and additional decreased by co-treatment with the TREM1 inhibitor LP17 (Fig. 8A–B). Transwell assays revealed that exosome therapy lowered microglial migration beneath inflammatory circumstances, which was additional inhibited by LP17 (Fig. 8C–D). EdU incorporation assays in BV2 cells demonstrated enhanced microglial proliferation in response to LPS + IFN-γ, which was lowered by Exos and much more so by LP17 (Fig. 8E–F). In vivo, EdU⁺Iba1⁺ proliferating microglia have been considerably elevated following tMCAO and decreased by hUMSC-Exos, with additional suppression noticed within the Exo + LP17 group (Fig. 8G). Furthermore, the proportion of CD16⁺EdU⁺Iba1⁺ pro-inflammatory proliferating microglia was additionally considerably lowered by Exo and LP17 therapies (Fig. 8H). These findings have been confirmed in vitro utilizing EdU labeling in main microglia (Fig. 8I) and thru immunofluorescence of each mind tissues (Fig. 8J) and cultured cells (Fig. 8Ok), the place LP17 and hUMSC-Exos synergistically attenuated inflammatory proliferation.
Collectively, these outcomes determine TREM1 as a important mediator of microglial inflammatory activation, migration, and proliferation after ischemic stroke, and reveal that hUMSC-Exos exert their therapeutic results by concentrating on TREM1 signaling. Pharmacological inhibition of TREM1 with LP17 additional amplifies the useful results of hUMSC-Exos, underscoring their potential as a combinatorial technique for post-stroke immunomodulation.
hUMSC-derived exosomes suppress microglial migration and activation by modulating TREM1-associated inflammatory networks. A Pattern evaluation of gene expression in microglia throughout Sham, tMCAO, and tMCAO + Exo teams recognized eight profiles. B Profile 5 represents 191 genes with a “low-high-low” expression development—upregulated in tMCAO and suppressed after Exo therapy. C Heatmap of Profile 5 genes reveals reversal of tMCAO-induced gene activation after hUMSC-Exo administration. D GO enrichment evaluation of Profile 5 genes reveals important involvement in cell migration, chemotaxis, and inflammatory response. E STRING-based protein-protein interplay community evaluation highlights TREM1 as a central hub within the regulation of inflammatory and migratory genes. F Heatmap of consultant Profile 5 genes together with Trem1, Cxcl2, Cxcl3, and Mmp9. G Immunofluorescence photographs of mind sections at day 3 post-tMCAO displaying co-localization of Iba1, CD16, and Trem1. H Quantification of Trem1⁺Iba1⁺ microglia confirms important suppression within the tMCAO + Exo group. I Confocal photographs of main microglia (pMG) displaying lowered Trem1 expression following Exo therapy in LPS + IFN-γ-activated cells. J qRT-PCR evaluation of Trem1 mRNA in pMG beneath the identical circumstances confirms suppression by Exos. Ok qRT-PCR of remoted microglia from mouse brains reveals Exo therapy downregulates Trem1, Cxcl2, and Cxcl3 after stroke. N = 4–6 per group. Scale bars as indicated. One-way ANOVA with Tukey’s submit hoc check. Information are offered as imply ± SEM. **p < 0.01, and ***p < 0.001
hUMSC-derived exosomes and TREM1 inhibition synergistically suppress microglial irritation, migration, and proliferation in vitro and in vivo. A–B Circulate cytometry histograms and quantification of COX-2, TNF-α, and iNOS expression in BV2 microglia handled with LPS + IFN-γ, with or with out hUMSC-Exos and/or the TREM1 inhibitor LP17. Exosome therapy markedly reduces inflammatory markers, with additional suppression by LP17. C–D Consultant photographs and quantification of Transwell migration assay present elevated migratory capability in LPS + IFN-γ-treated BV2 cells, which is lowered by hUMSC-Exos and additional inhibited by LP17. N = 9 per group. E–F Circulate cytometry plots and quantification of EdU incorporation in BV2 microglia point out elevated proliferation following LPS + IFN-γ stimulation, which is attenuated by hUMSC-Exos and additional suppressed by LP17. G–I In vivo and vitro evaluation present that hUMSC-Exos cut back the proportion of EdU⁺Iba1⁺ proliferating microglia, and CD16⁺EdU⁺Iba1⁺ pro-inflammatory microglia, with extra reductions upon LP17 therapy. J Immunofluorescence photographs from peri-infarct cortex of tMCAO mice displaying EdU, CD16, and Iba1 co-staining. Ok EdU incorporation in main microglia in vitro confirms that Exo and LP17 therapies suppress LPS + IFN-γ-induced microglial proliferation. N = 3–6 per group. Scale bars as indicated. Information are offered as imply ± SEM. **p < 0.01, ***p < 0.001 by ANOVA t-test
hUMSC-Exos suppress TREM1-mediated irritation by way of NF-κB and p38 MAPK inhibition
To research the molecular mechanisms by which hUMSC-Exos regulate microglial activation, we first assessed the influence of NF-κB signaling on TREM1-driven irritation. In LPS + IFN-γ-stimulated BV2 microglia, expression of pro-inflammatory genes together with Trem1, TNFα, IL1β, and IL6 was considerably upregulated. Remedy with hUMSC-Exos markedly lowered these inflammatory transcripts, whereas co-treatment with the NF-κB inhibitor BAY 11-7082 additional enhanced this suppression (Fig. S6). Conversely, the anti-inflammatory markers Arg1 and IL10 have been upregulated by Exos and additional elevated with BAY (Fig. S6), indicating that hUMSC-Exos mitigate irritation partly by way of NF-κB inhibition.
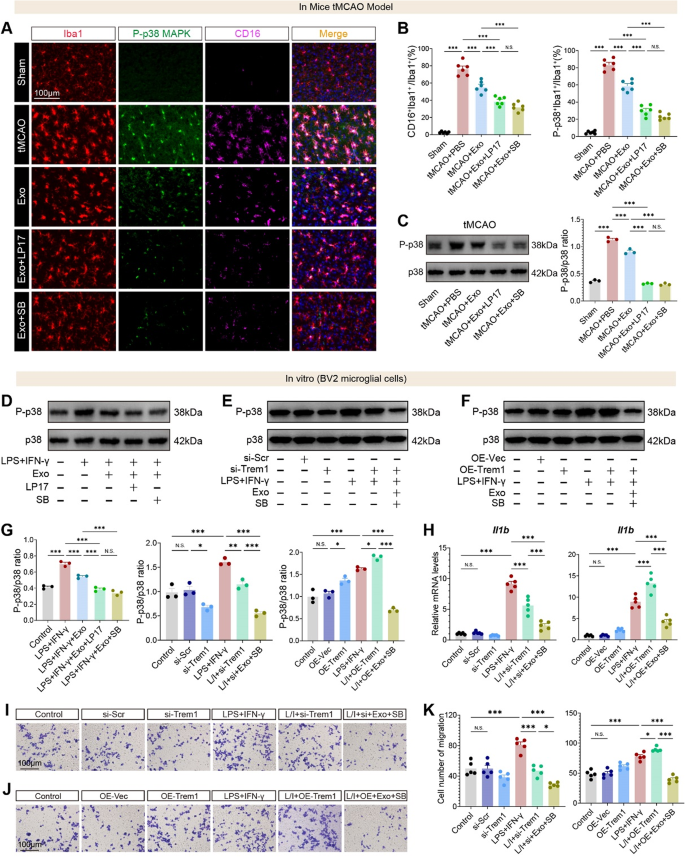
We subsequent evaluated the function of TREM1 in regulating p38 MAPK exercise. In vivo, immunofluorescence staining in tMCAO brains revealed a big enhance in CD16⁺Iba1⁺ pro-inflammatory microglia and p-p38⁺Iba1⁺ activated microglia, each of which have been decreased by hUMSC-Exos and additional suppressed by LP17 or SB203580 (p38 MAPK inhibitor) (Fig. 9A–B). Western blot evaluation of tMCAO mind tissues demonstrated that p38 MAPK phosphorylation was considerably elevated post-stroke and lowered by hUMSC-Exo therapy, with LP17 and SB203580 additional suppressing p-p38 ranges (Fig. 9C). To evaluate the useful penalties of p38 MAPK inhibition, we evaluated microglial migration utilizing Transwell assays. In vitro, hUMSC-Exos considerably suppressed LPS + IFN-γ-induced migration of BV2 microglia, and co-treatment with LP17 or SB203580 additional lowered migration with out additive results between the inhibitors (Fig. S7A-B).
Constant outcomes have been noticed for p38 MAPK activation: in BV2 microglia stimulated with LPS + IFN-γ, Exo therapy lowered p-p38 ranges, and suppression was enhanced by LP17 or SB203580 (Fig. 9D, G). To verify the efficacy of TREM1 genetic modulation, we assessed protein ranges following plasmid-mediated overexpression and siRNA knockdown in BV2 microglial cells. Western blot evaluation revealed that OE-TREM1 markedly elevated TREM1 protein expression by roughly threefold relative to controls (Fig. S8A), whereas si-TREM1 achieved a ~ 70% discount in expression (Fig. S8B), confirming efficient gain- and loss-of-function manipulations. Functionally, TREM1 knockdown abrogated LPS/IFN-γ-induced p38 MAPK activation, whereas TREM1 overexpression sustained p38 phosphorylation even within the presence of Exos (Fig. 9E–G). Moreover, Il1b expression was considerably decreased by hUMSC-Exos and additional lowered by both TREM1 knockdown or p38 inhibition (Fig. 9H). Conversely, OE-TREM1 rescued Il1b expression in Exo-treated cells except SB203580 was co-applied, suggesting that p38 MAPK acts downstream of TREM1 (Fig. 9H). Transwell assays confirmed that Exo therapy suppressed LPS + IFN-γ-induced migration, which was additional lowered by LP17, TREM1 knockdown, or p38 MAPK inhibition (Fig. 9I–Ok).
Collectively, these information set up a mechanistic axis wherein hUMSC-derived exosomes suppress TREM1 expression by way of NF-κB inhibition and consequently downregulate TREM1-mediated activation of the p38 MAPK pathway, thereby mitigating microglial inflammatory activation and migration.
hUMSC-Exos inhibit TREM1-mediated activation of the p38 MAPK pathway to suppress microglial irritation and migration. A Immunofluorescence photographs of Iba1⁺ microglia co-stained with CD16 and phosphorylated p38 MAPK (P-p38) in tMCAO brains handled with Exos ± LP17 or SB203580. B Quantification of CD16⁺Iba1⁺ cells and P-p38⁺Iba1⁺ cells. C Western blot evaluation of P-p38 and complete p38 in tMCAO mind tissues beneath indicated therapies. D Western blot of P-p38 and p38 in LPS + IFN-γ-stimulated BV2 microglia ± Exos, LP17, or SB203580. E–F Western blot of P-p38 in BV2 cells with TREM1 knockdown (E) or overexpression (F) ± Exos or SB203580. G Quantification of P-p38/p38 ratio from blots in panels D–F. H qRT-PCR for Il1b in TREM1 knockdown (left) and OE (proper) BV2 cells ± Exos or SB203580. I–J Transwell migration photographs of BV2 cells with TREM1 knockdown (I) or overexpression (J). Ok Quantification of migrated cells per subject. N = 3–6 per group. Scale bars as indicated. Information are offered as imply ± SEM. *p < 0.05, **p < 0.01, and ***p < 0.001. “ns” signifies no important distinction
hUMSC-Exos promote neuroprotection and useful restoration by way of HMGB1-mediated suppression of TREM1-p38 MAPK signaling
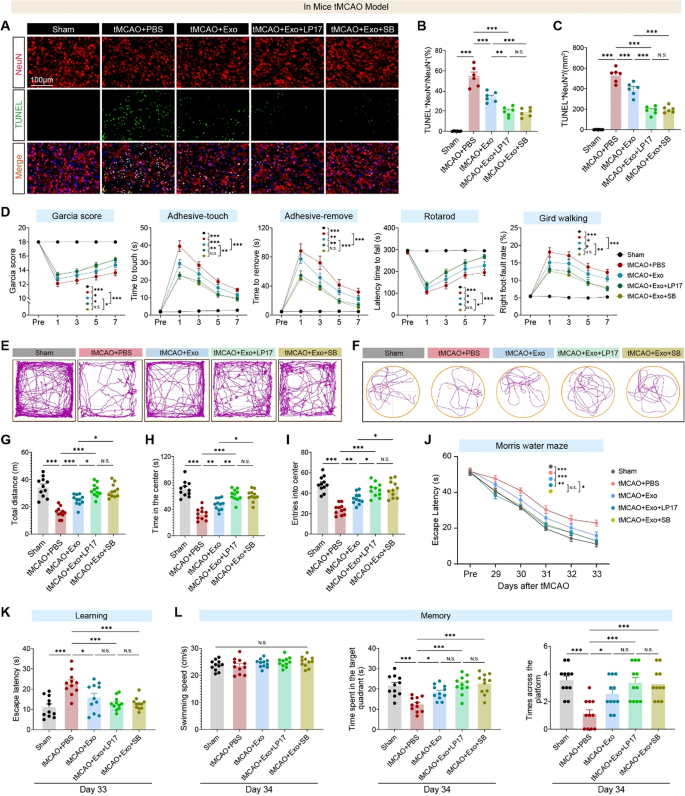
Neuronal apoptosis within the peri-infarct cortex of tMCAO mice was first assessed to guage the therapeutic potential of hUMSC-Exos in ischemic stroke. Immunofluorescence staining revealed a big enhance in TUNEL⁺NeuN⁺ apoptotic neurons following stroke, with notable discount within the hUMSC-Exo-treated group. Co-administration of LP17 or SB203580 additional decreased apoptosis, suggesting Exos exert neuroprotection by inhibiting the TREM1–p38 MAPK axis (Fig. 10A–C). Behavioral assessments demonstrated important enhancements in sensorimotor operate with hUMSC-Exo therapy: Exo-treated mice confirmed higher Garcia scores, sooner adhesive removing responses, improved rotarod coordination, and fewer grid-walking foot-faults. Notably, co-treatment with LP17 or SB203580 enhanced these results (Fig. 10D), highlighting the function of TREM1–p38 signaling in post-stroke motor impairments. In open subject checks, tMCAO mice exhibited anxiety-like and hypoactive behaviors (lowered motion, central zone avoidance), which have been reversed by hUMSC-Exos; LP17 or SB203580 co-treatment additional enhanced exploratory conduct (Fig. 10E–I), suggesting TREM1–p38 MAPK inhibition alleviates stroke-induced affective disturbances by reshaping the neuroimmune microenvironment.
Lengthy-term cognitive restoration was assessed utilizing Morris Water Maze testing on days 29–34 post-stroke. In the course of the acquisition section, tMCAO mice confirmed extended escape latencies, which have been shortened by hUMSC-Exo therapy; co-treatment with LP17 or SB203580 additional improved spatial studying, emphasizing TREM1’s function in studying acquisition (Fig. 10J-Ok). Within the day 34 probe trial, Exo-treated mice demonstrated superior reminiscence retention (extra time in goal quadrant, better platform zone distance, frequent crossings), with LP17 or SB203580 co-treatment restoring efficiency to near-sham ranges (Fig. 10L). This means each TREM1 and p38 MAPK signaling contribute to post-stroke spatial reminiscence consolidation.
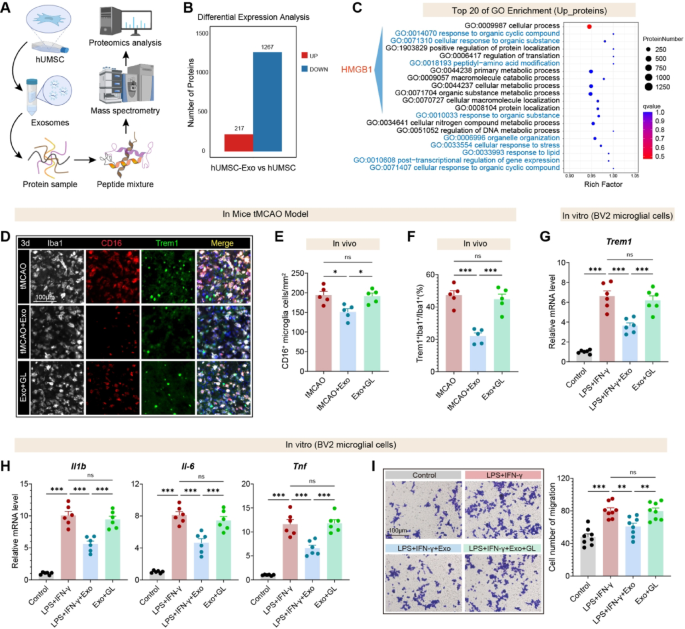
Molecular mediators of hUMSC-Exos’ immunomodulatory results have been recognized by way of complete proteomic comparability between hUMSC-Exos and parental hUMSCs. This evaluation revealed 1,484 differentially expressed proteins, with 217 considerably enriched in exosomes—in keeping with selective loading of immunoregulatory effectors (Fig. 11A–B). GO enrichment evaluation highlighted pathways linked to immune modulation and stress responses, with distinguished HMGB1-related organic processes (Fig. 11C), implicating HMGB1 as a possible mediator.
To functionally assess the relevance of HMGB1, glycyrrhizin (GL), a pharmacological inhibitor of HMGB1, was co-administered with hUMSC-Exo in tMCAO mouse mannequin. Immunofluorescence staining revealed that hUMSC-Exo considerably lowered the variety of CD16⁺ and TREM1⁺ microglia within the peri-infarct area (Fig. 11D), as quantified in Fig. 11E–F. Notably, GL co-treatment partially reversed this suppression, suggesting that HMGB1 acts upstream of TREM1 to control microglial activation in vivo.
In vitro validation used LPS/IFN-γ-stimulated BV2 microglia handled with hUMSC-Exos ± GL. hUMSC-Exos markedly suppressed Trem1 transcripts, whereas GL abrogated this impact (Fig. 11G). Equally, hUMSC-Exos attenuated pro-inflammatory cytokines (Il1b, Il6, Tnf), with GL mitigating this suppression (Fig. 11H). Transwell assays confirmed lowered microglial migration with hUMSC-Exos, an impact reversed by GL (Fig. 11I). Collectively, these information assist a mannequin the place hUMSC-Exos inhibit HMGB1–TREM1 signaling to suppress microglial activation, cytokine launch, and migration, contributing to post-stroke neuroprotection.
hUMSC-derived exosomes attenuate neuronal apoptosis and restore motor, emotional, and cognitive operate in tMCAO mice by way of TREM1 and p38 MAPK pathway suppression. A Consultant immunofluorescence photographs of NeuN (purple) and TUNEL (inexperienced) staining within the peri-infarct cortex at day 3 post-tMCAO. Scale bar: 100 μm. B–C Quantification of TUNEL⁺NeuN⁺ apoptotic neurons (n = 6 per group). D Behavioral efficiency over time: tMCAO considerably impairs motor/sensory capabilities as assessed by Garcia rating, adhesive-touch/removing checks, rotarod, and grid strolling, that are restored by hUMSC-Exos and additional improved by LP17 or SB203580 (n = 11 mice/group). E–F Consultant locomotor traces from the open subject and Morris water maze check point out lowered exploratory exercise and impaired search conduct in tMCAO + PBS mice, improved with hUMSC-Exo or mixed therapies. G–I Quantification of open subject check outcomes: complete distance traveled (G), time spent in heart (H), and heart entries (I) are all lowered after stroke and considerably restored by Exo, with extra profit from LP17 or SB203580. N = 11 mice/group. J MWM coaching (days 29–33 post-tMCAO): escape latency is considerably extended in stroke mice and shortened with Exo or Exo + LP17/SB therapy, indicating enhanced spatial studying. N = 11 mice/group. Ok On day 33, Exo-treated mice exhibit considerably improved escape latency, significantly together with LP17. L Probe trial (day 34) assessing reminiscence retention: Exo therapy will increase time within the goal quadrant, swimming tracks throughout the platform, and the variety of platform crossings. These cognitive enhancements are additional enhanced by LP17 and SB203580. Information symbolize imply ± SEM. One-way or two-way ANOVA adopted by Tukey’s submit hoc check was used for a number of comparisons. NS = not important, *p < 0.05, **p < 0.01, ***p < 0.001
hUMSC-derived exosomes attenuate microglial irritation by way of HMGB1-Trem1 signaling. A Schematic workflow of proteomic evaluation evaluating hUMSC-Exo and hUMSC whole-cell lysates, utilizing mass spectrometry to determine differentially expressed proteins. B Bar graph displaying 217 proteins considerably upregulated and 1267 downregulated in hUMSC-Exo in comparison with hUMSC. C Gene Ontology (GO) enrichment evaluation of upregulated proteins. The highest 20 GO phrases embrace processes associated to inflammatory signaling, protein modification, and mobile response to emphasize, with excessive enrichment of HMGB1-associated pathways. D Consultant immunofluorescence photographs displaying Iba1⁺ microglia co-stained with CD16 (pro-inflammatory marker) and TREM1 within the peri-infarct cortex at 3 days post-tMCAO. Scale bar, 100 μm. E–F Quantification of CD16⁺ microglial density (E) and TREM1⁺/Iba1⁺ microglial ratio (F) confirms anti-inflammatory results of hUMSC-Exo and reversal by GL (n = 6 per group). G qRT-PCR evaluation in BV2 microglial cells reveals Trem1 mRNA ranges. H Expression of Il1b, Il6, and Tnf is considerably suppressed by hUMSC-Exo and partially restored with GL in LPS + IFN-γ-stimulated BV2 cells. I Transwell migration assay demonstrates lowered migration of activated BV2 microglia upon hUMSC-Exo therapy, with partial restoration upon GL co-treatment. Scale bars: 100 μm. Information are offered as imply ± SEM. One-way ANOVA with Tukey’s submit hoc check. *p < 0.05, **p < 0.01 and ***p < 0.001. “ns” signifies no important distinction