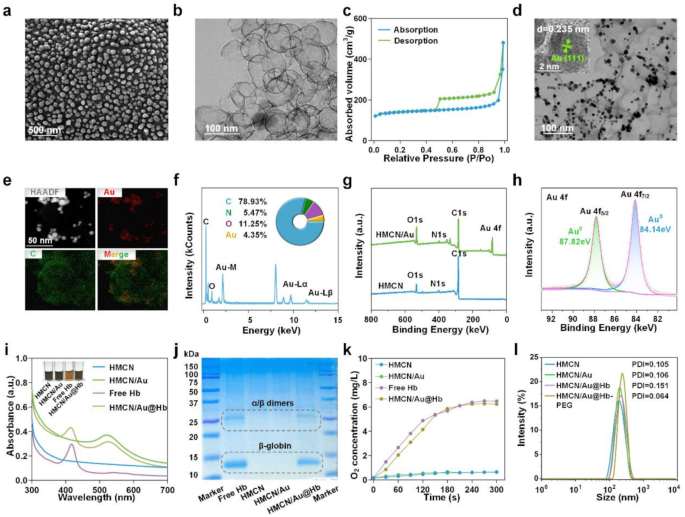
Preparation and characterization of HMCN/Au@Hb
Initially, HMCN was fabricated as a core–shell construction utilizing a modified hard-templating technique. Scanning electron microscopy (SEM) photos revealed that HMCN have been spherical, uniformly distributed, and had a median diameter of 57.06 ± 10.2 nm (Fig. 1a). Transmission electron microscopy (TEM) additional demonstrated that the HMCN possessed an inside hole construction alongside with a median ultrathin shell thickness of two.35 nm (Fig. 1b). Brunauer Emmet Teller (BET) N2 absorption-desorption evaluation confirmed the presence of plentiful voids with pore dimension of 1.76 nm and a excessive particular floor space as much as 532.7475 m2/g, contributing to the improved loading capability of HMCN (Fig. 1c and Determine S1). After bodily absorption of Au onto the floor of HMCN, Au introduced as protrusions in TEM photos, like chocolate beans on cookies. Au and HMCN have been thus tightly built-in and HMCN/Au was efficiently fabricated (Fig. 1d). Moreover, the lattice airplane spacing of 0.235 nm measured via the high-resolution TEM (HRTEM) picture corresponds to the (111) diffraction airplane of Au (Fig. 1d insert). Concurrently, the profitable ornament of Au was proved by mapping aspect profiles (Fig. 1e), and the coexistence of Au and C parts within the HMCN/Au have been additional validated by energy-dispersive X-ray spectrometer (EDS) (Fig. 1f). Afterwards, X-ray photoelectron spectroscopy (XPS) was carried out to measure the aspect composition and valence states of HMCN/Au (Fig. 1g). The high-resolution Au 4f spectrum will be deconvoluted into two doublets centered at 87.82 and 84.14 eV, labeled as Au4f5/2 and Au4f7/2, respectively, which indicated that Au precursor was partially decreased to metallic Au (Fig. 1h) [36]. As well as, the chosen space electron diffraction (SAED) sample with the (111), (200), (220) and (311) planes of cubic HMCN/Au echo the outcomes from HRTEM (Determine S2).
Preparation and characterization of HMCN/Au@Hb. (a) SEM and (b) TEM photos of HMCN. (c) N2 absorption/desorption isotherms of HMCN. (d) TEM photos of HMCN/Au (Insert was HRTEM). (e) HAADF-STEM picture and elemental mapping for HMCN/Au. (f) Quantitative elemental evaluation of HMCN/Au carried out utilizing EDS. The inserts have been the comprised aspect and corresponding atomic frequencies. (g) XPS evaluation of the HMCN and HMCN/Au. (h) The high-resolution XPS spectra of Au 4f for HMCN/Au. (i) UV-VIS spectra of free Hb, HMCN, HMCN/Au and HMCN/Au@Hb, respectively (Insert was the colour change earlier than and after loading). (j) Coomassie blue staining carried out on electrophoresed acrylamide loaded with Hb. (okay) Oxygen releasing properties measured by RDPP probe. (l) Dimension distribution of HMCN, HMCN/Au, HMCN/Au@Hb and PEGylated HMCN/Au@Hb decided by DLS.
Given to the hole and mesopores of HMCN/Au, Hb was effectively loaded and HMCN/Au@Hb was ultimately fashioned. The UV-VIS spectra of HMCN/Au@Hb exhibited attribute absorption peaks at 410 and 525 nm similar to Hb and Au (Fig. 1i). Furthermore, the insert picture illustrates the colour adjustments throughout the synthesis of HMCN/Au@Hb, the darkish grey HMCNs become a barely brownish darkish gray coloration after loaded with Hb (Fig. 1i insert). Coomassie blue staining revealed protein bands at 14 and 25 kDa, in correlation with the β-globin and α/β dimers of Hb (Fig. 1j) [37]. Based mostly on the usual focus curve of Hb (Determine S3a, b), the optimum mass ratio was decided to be 1:5 (Hb: HMCN) (Desk S1). The oxygen-carrying capability of various nanomaterials was then assessed utilizing a dissolved-oxygen meter. Specifically, a major rise in O2 ranges was detected in free Hb and HMCN/Au@Hb, which confirmed that Hb retained its oxygen-carrying exercise throughout the synthesis course of (Fig. 1okay). Furthermore, the floor of HMCN/Au@Hb have been modified with DSPE-PEG2000-NH2 to reinforce the biocompatibility and stability in physiological situation. Dynamic gentle scattering (DLS) evaluation confirmed a barely elevated particle dimension following Au ornament, Hb loading and PEGylation. The hydrodynamic sizes of HMCN, HMCN/Au, HMCN/Au@Hb and PEGylated HMCN/Au@Hb dispersed in deionized water have been measured as 177.57 ± 5.10 nm (PDI = 0.105), 205.50 ± 2.50 nm (PDI = 0.106), 228.67 ± 3.05 nm (PDI = 0.151) and 259.77 ± 4.95 nm (PDI = 0.064), respectively (Fig. 1l), with a uniform dimension distribution. In abstract, the profitable preparation of HMCN/Au@Hb was confirmed via aspect composition willpower, morphological statement and spectral characterization.
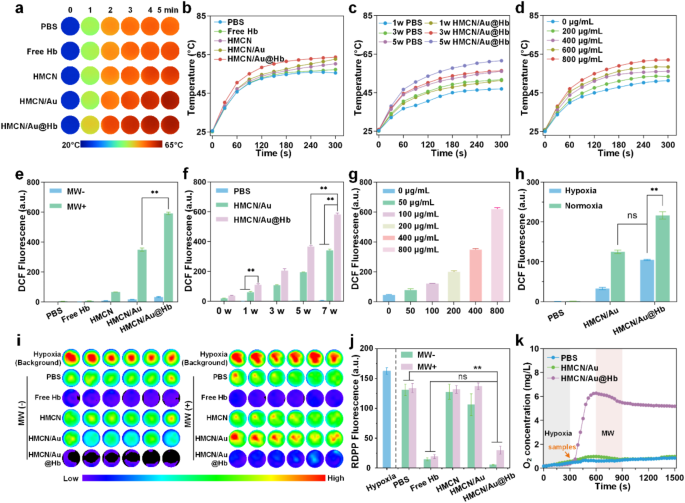
Microwave responsive and oxygen carrying capacities of HMCN/Au@Hb
Contemplating HMCN displays excessive dielectric constants on account of its distinctive porous construction and conductive carbon framework, the microwave-thermal efficiency of HMCN/Au@Hb was monitored underneath managed microwave parameters, together with energy density and nanocomposites focus [38]. Following a 5-min microwave irradiation (3.0 W), the management and free Hb group solely elevated to 55.5 and 57.2℃, respectively. Whereas underneath the identical irradiation situation, HMCN, HMCN/Au and HMCN/Au@Hb (400 µg/mL) have been heated as much as 60.2, 62.6 and 63.7℃, respectively, the ultimate temperature of HMCN/Au@Hb was about 8.2℃ larger than the temperature of PBS. These outcomes exhibit that HMCN/Au@Hb contributed to enhancing microwave-to-heat conversion effectivity to a sure extent (Fig. 2a) (corresponding infrared thermal picture was introduced in Fig. 2b). Moreover, HMCN/Au@Hb was irradiated with microwave underneath totally different energy densities, the temperature and the microwave energy density have been positively correlated (Fig. 2c) (corresponding infrared thermal picture was introduced in Determine S4a). Furthermore, upon publicity to microwave (3.0 W), the temperature elevated from 51.3 to 53.4, 56.1, 58.4 and 62.0℃ with growing focus of HMCN/Au@Hb (0, 200, 400, 600 and 800 µg/mL) (Fig. 2d), indicating that the microwave-thermal impact of HMCN/Au@Hb was proportional to the pattern focus (corresponding infrared thermal picture was introduced in Determine S4b). These findings spotlight the potential of HMCN/Au@Hb as a wonderful microwave absorption agent to enhance the microwave-thermal conversion effectivity for tumors.
Microwave responsive and oxygen carrying capacities of HMCN/Au@Hb. (a) Actual-time infrared thermal photos and (b) corresponding heating profiles of various samples (400 µg/mL) underneath microwave irradiation (3 W, 5 min). (c) Microwave heating profiles of PBS and HMCN/Au@Hb (400 µg/mL) underneath microwave irradiation at totally different energy densities for five min. (d) Microwave heating profiles of HMCN/Au@Hb at totally different concentrations underneath microwave irradiation (3 W, 5 min). (e) ROS detection of various samples (200 µg/mL) no matter microwave irradiation (7 W, 5 min). (f) ROS detection of various samples (200 µg/mL) underneath microwave irradiation (5 min) at totally different energy ranges. (g) ROS detection of HMCN/Au@Hb suspensions at totally different concentrations underneath microwave irradiation (3 W, 5 min). (h) ROS detection of various samples (PBS, HMCN/Au and HMCN/Au@Hb) on the focus of 200 µg/mL underneath hypoxic or normoxic situations underneath microwave irradiation (3 W, 5 min). (i) Fluorescence photos and (j) quantitative analyses of various samples displaying oxygen era underneath totally different situations. (okay) Dynamic oxygen era of various samples underneath microwave irradiation (3 W, 5 min). Information have been introduced as imply values ± SD (n = 3). (* represents p<0.05, ** represents p<0.01)
Impressed by the robust SPR attribute of Au and the enough oxygen launched by Hb, we inferred that Au may generate lively or “sizzling” electrons in order to induce electron’s transition upon microwave irradiation and additional take in oxygen molecules as sustainable gasoline for ROS era [39]. To research whether or not the HMCN/Au@Hb possessed the flexibility of microwave-dynamic efficiency, the manufacturing and species willpower of ROS underneath microwave irradiation have been carried out via electron spin resonance (ESR). Notably, the HMCN/Au@Hb + MW group exhibited a attribute sextet sign similar to superoxide anions (•O2−), which was remarkably larger than the HMCN/Au + MW group. The end result indicated that Hb would possibly play an important function in oxygen-amplified microwave-dynamic efficiency (Determine S5). We then used the DCFH-DA probe to detect complete ROS era of HMCN/Au@Hb. Upon microwave irradiation (7.0 W), the fluorescence depth of HMCN/Au@Hb was virtually 1.7 occasions larger than that of HMCN/Au, indicating the constructive impact of oxygen in amplifying ROS era (Fig. 2e). Subsequently, the fluorescence depth of PBS, HMCN/Au and HMCN/Au@Hb confirmed a equally power-dependent improve. Underneath the identical energy density, the HMCN/Au@Hb group exhibited larger fluorescence depth in comparison with that of the HMCN/Au group (Fig. 2f). Furthermore, the ROS era of HMCN/Au@Hb confirmed a concentration-dependent development (Fig. 2g). Subsequently, we believed that the oxygen carried by HMCN/Au@Hb facilitated the amplification of ROS era. After irradiated with 3.0 W microwave, the ROS indicators of HMCN/Au in normoxic atmosphere was 1.7 occasions larger than that in hypoxic atmosphere, however akin to HMCN/Au@Hb in the identical hypoxic atmosphere (Fig. 2h). The end result demonstrated that Hb would possibly complement the O2 content material in hypoxic atmosphere, which might additional facilitate the oxidation course of.
To additional discover the inside oxygen-carrying property of HMCN/Au@Hb, we employed an oxygen indicator, RDPP probe, which will be quenched by molecular oxygen, to visually examine the oxygen manufacturing [40]. Notably, the fluorescence depth within the PBS and HMCN teams displayed a negligible change. Nevertheless, the fluorescence depth in each the free Hb and HMCN/Au@Hb group weakened dramatically, suggesting the equally superior O2 era actions of each the free Hb and HMCN/Au@Hb (Fig. 2i), which was according to the corresponding quantitative knowledge introduced in Fig. 2j. In addition to, each PBS and HMCN/Au contained little or no oxygen and remained unchanged after irradiating with microwave, whereas HMCN/Au@Hb (3 mg/mL) confirmed instant improve within the O2 focus at a hypoxic resolution and maintained the extraordinarily excessive degree of O2 throughout the means of microwave irradiation (Fig. 2okay). The above outcomes confirmed the oxygen-carrying exercise of HMCN/Au@Hb, in addition to a small quantity of oxygen consumption to generate ROS underneath the publicity of microwave.
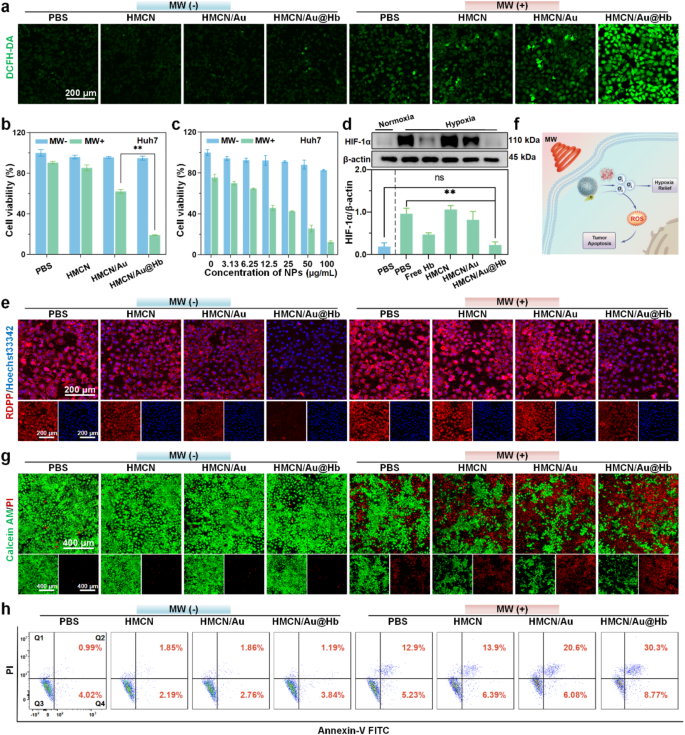
Cytotoxicity of HMCN/Au@Hb underneath microwave irradiation
Impressed by the ROS era property on the resolution degree, we additional evaluated HMCN/Au@Hb induced ROS outbreak on the mobile degree. DCFH-DA probe was utilized to visualise and characterize the ROS era through confocal laser scanning microscope (CLSM) [41]. Herein, invisible inexperienced fluorescence was detected in cells handled with PBS or NPs alone, whereas extra intense inexperienced fluorescence was proven upon microwave irradiation. Furthermore, the brightest inexperienced fluorescence was noticed within the HMCN/Au@Hb + MW group (Fig. 3a). Moreover, the quantitative knowledge throughout totally different teams discovered that the ROS ranges within the HMCN/Au@Hb + MW group have been roughly 1.8-fold larger than that of the HMCN/Au + MW group, indicating that intrinsic oxygen-carrying capability of Hb would possibly induce a drastically enhanced microwave-dynamic impact (Determine S6a). Subsequently, the aforementioned cells have been harvested for circulation cytometry evaluation, and confirmed a constant development in comparison with these noticed in DCFH-DA assay (Determine S6b). These findings immediately highlighted the effectiveness of HMCN/Au@Hb in producing ROS triggered by microwave irradiation. We additional utilized higher transwell chamber to imitate “transition zone” to analyze whether or not tumor cells within the marginal “transition zone” may nonetheless trigger ROS burst underneath ultra-low microwave irradiation, Though the temperature within the higher chamber of the microwave irradiation group was considerably decrease than that within the backside chamber and direct heating group, the ROS ranges have been akin to these within the direct heating group. It additional demonstrated that even underneath low-power microwave irradiation within the “transition zone”, nanoparticles may nonetheless be successfully excited to provide ROS (Determine S7).
In vitro microwave-induced cytotoxic impact. (a) CLSM photos of intracellular ROS degree with totally different remedies (inexperienced, fluorescence of DCFH-DA). (b) Cytotoxicity of Huh-7 cells handled with totally different samples and (c) handled with HMCN/Au@Hb at totally different concentrations. (d) Western blot demonstrating the HIF-1α expression ranges with totally different remedies. (e) CLSM photos of intracellular O2 degree with totally different remedies (blue, fluorescence of Hoechst 33342; crimson, fluorescence of RDPP; overlay photos). (f) Schematic illustration of HMCN/Au@Hb mediated microwave thermal-dynamic impact. (g) Dwell/Lifeless co-staining and (h) Annexin V/PI staining of Huh-7 cells after totally different remedies. Information have been introduced as imply values ± SD (n = 3). (* represents p<0.05, ** represents p<0.01)
On condition that extreme oxidative stress detrimentally impacts the mobile killing impact, the antitumor impact of HMCN/Au@Hb in Huh-7 cells was initially characterised by MTT assay [42]. After being irradiated with microwave, the cell viability price reveals an roughly 80% discount in MW + HMCN/Au@Hb (50 µg/mL) group, which was larger than that in MW + HMCN/Au (50 µg/mL) group (Fig. 3b). Particularly, cell viability was regularly decreased within the HMCN/Au@Hb + MW group with growing concentrations of HMCN/Au@Hb, supporting its successfully antitumor impact in a concentration-dependent method. Against this, insignificant cytotoxic impact was noticed in non-irradiated situations, suggesting the noncytotoxic nature of HMCN/Au@Hb (Fig. 3c). To additional examine the cytotoxicity of HMCN/Au@Hb in regular cell strains, human regular hepatic cell (LO2 and MIHA) and renal cell (293T) have been used within the following experiments. Even at a particularly excessive focus of 400 µg/mL, 3 sorts of cell line remained viability of 76.4%, 85.6% and 80.7%, respectively, indicating the commendable biosafety of HMCN/Au@Hb (Determine S8).
Because of the enough O2 launched by HMCN/Au@Hb, we speculated that the inherent oxygen ranges would considerably elevate and mitigate the constraints imposed by the hypoxic microenvironment, thereby facilitating ROS manufacturing and amplifying the therapeutic impact [43]. Preliminary investigation of the hypoxia-relieving impact in vitro, western blot was carried out to detect the expression degree of HIF-1α [44]. HIF-1α expression degree was considerably down-regulated within the HMCN/Au@Hb group however akin to that of the damaging management (PBS underneath normoxic situations), solidly verifying that each Hb and HMCN/Au@Hb possessed equal hypoxia-allaying results (Fig. 3d). Subsequently, to analyze the dynamic means of oxygen variation, mobile O2 content material was analyzed by CLSM statement utilizing the RDPP indicator. After incubated with HMCN/Au@Hb, the crimson fluorescence depth was weakened acutely in comparison with that of the PBS group, suggesting a considerable improve in mobile oxygen ranges. Nonetheless, the crimson fluorescence within the HMCN/Au@Hb + MW group was barely stronger than that within the HMCN/Au@Hb group (Fig. 3e). Fluorescence semi-quantitative (Determine S9a) and circulation cytometry measurements (Determine S9b) additional confirmed that oxygen consumption could happen via producing ROS upon microwave stimulus. Altogether, owing to the particular hypoxic nature of tumor tissue, we speculated that the enough oxygen launched by Hb-loaded HMCN/Au would possibly facilitate ROS era in vitro, thereby ameliorating the hypoxic microenvironment and augmenting the therapeutic efficacy (Fig. 3f) [45].
As well as, the antitumor impact induced by extreme ROS and microwave hyperthermia was assessed utilizing Calcein-AM/PI co-staining. Ultralow cell demise charges have been noticed in non-irradiated teams. After irradiation, cell demise charges elevated dramatically, and cells incubated with HMCN/Au@Hb exhibited the best demise price, which was conformed to the findings of the MTT assay (Fig. 3g). The fluorescence quantitative outcomes additionally remarkably emphasised the numerous outcomes of the MW coupling system (Determine. S10). Consequently, cell apoptosis assay was carried out on Huh-7 cells to establish the kind of apoptosis triggered by ROS and hyperthermia. Cells handled with HMCN/Au + MW and HMCN/Au@Hb + MW displayed a considerable improve within the proportion of apoptotic (Annexin V-positive) and necroptotic cells (PI-positive) (Fig. 3h). Particularly, apparent apoptosis indicators (Q2 + This autumn quadrant: 39.1%) have been noticed within the HMCN/Au@Hb + MW group, which was superior to that of the HMCN/Au + MW group (Q2 + This autumn quadrant: 26.7%) (Determine. S11).
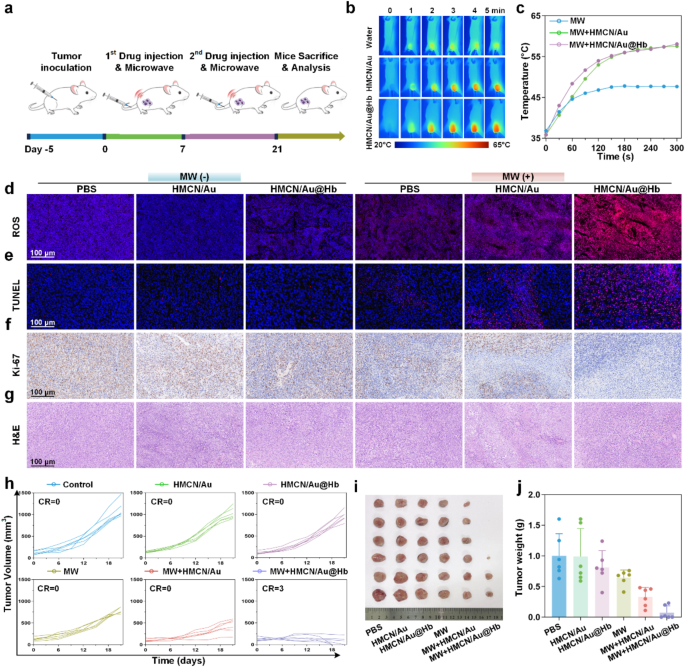
In vivo antitumor impact
Because of the exceptional in vitro cell demise impact, the in vivo antitumor results have been evaluated on tumor-bearing mice. The HCC fashions have been randomly divided into six teams (n = 6): PBS, HMCN/Au, HMCN/Au@Hb and their respective MW teams. Determine 4a confirmed your entire in vivo experimental course of. All through the therapy, in vivo infrared thermal photos (Fig. 4b) and corresponding temperature curves (Fig. 4c) revealed that tumor temperature within the MW + HMCN/Au and MW + HMCN/Au@Hb teams elevated quickly inside 5 min, even reached 58.1 °C and 57.6 °C underneath an ultralow microwave energy density (3.0 W), respectively. Nevertheless, the temperature of tumors with out NPs administration solely elevated to 47.7 °C, indicating that HMCN/Au and HMCN/Au@Hb exhibited a wonderful microwave-thermal impact in vivo on account of their related localized warmth accumulation skill. To additional consider the biodistribution of HMCN/Au@Hb, ICG labeled fluorescence imaging was carried out on Hepa1-6 tumor xenograft mice. Following intravenous injection of free ICG or HMCN/Au@Hb@ICG, fluorescence photos have been acquired at totally different timepoints (0, 1, 2, 4, 6, 8, 12 and 24 h). Each teams exhibited robust fluorescence sign at 1 h (Determine S12a). Notably, HMCN/Au@Hb demonstrated considerably larger fluorescence retention on the tumor website in comparison with free ICG, with secure tumor fluorescence persisting past 24 h, whereas the ICG group confirmed minimal sign past 8 h. This extended tumor accumulation was doubtless attributed to the optimum nanoparticle dimension, facilitating the improved permeability and retention (EPR) impact (Determine S12b). Ex vivo imaging additional confirmed sustained fluorescence in tumors, supporting the extended retention of HMCN/Au@Hb in tumor tissue (Determine S12c). As well as, we supplemented the Au content material in tumor and different organs with Inductively Coupled Plasma Mass Spectrometry (ICP-MS), it revealed that apparent Au enrichment within the tumor of HMCN/Au@Hb group on the time of sacrifice (1-hour post-injection), demonstrating the wonderful tumor accumulation potential. In addition to, it additionally demonstrated vital hepatic and renal uptake, presumably because of the major involvement of the liver and kidneys within the metabolic clearance of the nanomaterials (Determine S13). Nevertheless, ex vivo distribution of each free ICG and ICG-labeled HMCN/Au@Hb decreased quickly and was almost utterly cleared after 24 h administration, as beforehand proven in Determine S12c.
In vivo antitumor impact. (a) Schematic illustration of the experimental schedule for the subcutaneous tumor mannequin remedies. (b) Infrared thermal photos and (c) the corresponding heating profiles of tumor-bearing mice throughout microwave irradiation. Histological evaluation of sacrificed tumor tissues after a 21-day therapy (d) ROS, (e) TUNEL, (f) Ki-67 and (g) H&E staining. (h) Particular person of tumor development curves in numerous teams. (i) Images (j) and the corresponding weight of tumor tissues at 21st day after remedies. Information have been introduced as imply values ± SD (n = 6). (* represents p<0.05, ** represents p<0.01)
The potent antitumor functionality of HMCN/Au@Hb was additional investigated via ROS, TUNEL, Ki-67 and H&E staining of tumor tissues dissociated on day 21. Important variations within the morphology of tumors have been noticed throughout totally different therapy teams. Remarkably, HMCN/Au@Hb + MW handled tumors exhibited larger ranges of ROS era than the opposite teams, indicating that ROS outbreak could play a pivotal function in tumor suppression (Fig. 4d, Determine S14a). As well as, TUNEL staining confirmed that HMCN/Au@Hb + MW group exhibited essentially the most intense crimson fluorescence, indicative of apoptotic tumor cell, highlighting the superior microwave-dynamic property to induce most cancers cell apoptosis (Fig. 4e, Determine S14b). Equally, tumors stained with Ki-67 (Fig. 4f, Determine S14c) and H&E (Fig. 4g) revealed that HMCN/Au@Hb + MW displayed the bottom tumor cell proliferation and essentially the most in depth karyorrhectic particles, in sharp distinction to the plentiful proliferation and restricted necrosis noticed in different teams. These findings demonstrated that HMCN/Au@Hb achieved pronounced antitumor impact by inducing ROS outbreak upon microwave irradiation.
Inspired by the good efficiency of HMCN/Au@Hb in thermal imaging and tumor cell harm in vivo, adjustments in tumor quantity have been monitored all through 21-day therapy (Determine S15). In comparison with the quickly tumor development within the management, HMCN/Au, HMCN/Au@Hb and MW-only teams, solely average tumor inhibition noticed within the HMCN/Au + MW group, whereas the tumor development curves started to extend beginning on the fifteenth day, because the inadequate oxygen could produce insufficient microwave-dynamic impact and in the end result in incomplete tumor necrosis and recurrence. In the meantime, the HMCN/Au@Hb + MW group introduced with substantial tumor suppression, in addition to a major and definitive healing impact (Fig. 4h and Determine S16). Tumor pictures (Fig. 4i) and tumor weights (Fig. 4j) defined the same lead to a extra intuitive and clear approach. Moreover, the potential uncomfortable side effects have been evaluated throughout the 21-day statement interval. Notably, no vital fluctuations have been noticed in physique weight throughout all numerous teams, which preliminarily confirmed the wonderful biosafety of all remedies in mice (Determine S17). To additional examine potential thermal harm to regular tissues adjoining to tumor lesions post-ablation, we carried out pathological examinations. Histological analyses revealed a well-demarcated ablation zone, and there have been no apparent heating harm results in surrounding muscle tissues upon microwave irradiation (Determine S18). Furthermore, H&E staining was carried out on main organs throughout totally different handled mice, and no observable tissue necrosis was discovered. It urged that each nanomaterials administration and microwave irradiation had no seen pathological abnormalities or inflammatory lesions in vivo (Determine S19). Collectively, HMCN/Au@Hb exhibited superior tumoricidal impact and favorable biosafety, which was anticipated to be a biocompatible nanomaterial for microwave ablative remedy sooner or later.
To research the tumor recurrence, we additional carried out a numerous of experiment teams to repeatedly monitor the tumor development for 33 days (Word: tumor quantity ≥ 1500 mm3 outlined as useless). Comparative evaluation at day 7 post-ablation revealed that the MW + HMCN/Au@Hb group (V/V0 = 0.998) confirmed higher tumor inhibition impact than the MW-alone group (V/V0 = 1.555). Notably, some chosen tumor-bearing mice even achieved full tumor ablation in MW + HMCN/Au@Hb group. In distinction, the tumor quantity within the non-ablation group surpassed the moral endpoint threshold (≥ 1500 mm3), necessitating termination of statement on account of speedy development (Determine S20a). Extra encouragingly, there was no vital native recurrence within the MW + HMCN/Au@Hb group even at day 33 post-ablation (V/V0 = 0.414), in sharp distinction to the MW group (V/V0 = 7.481) (Determine S20b). As well as, the survival price of mice handled with HMCN/Au@Hb plus MW was considerably extended (Determine S20c). Subsequently, in comparison with microwave ablation alone, HMCN/Au@Hb considerably enhanced the efficacy of microwave ablation and successfully inhibited tumor recurrence at post-ablation.
Antitumor immune response
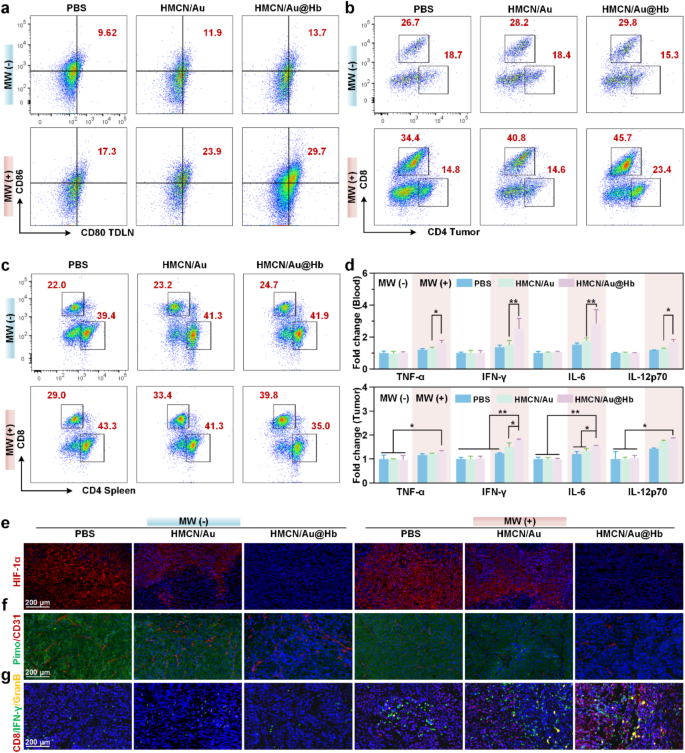
To additional examine the underlying mechanism of the antitumor impact of HMCN/Au@Hb, the activation of systemic immunity was explored [46]. Earlier research have proven that DCs, principally contained in tumor draining lymph nodes (TDLNs), acted as potent antigen-presenting cells (APCs) for capturing tumor related antigens (TAAs) [47]. Herein, we discovered that the HMCN/Au@Hb + MW group confirmed a larger matured DCs (CD80+CD86+) infiltration, which far exceeded that noticed within the HMCN/Au + MW group (Fig. 5a). Quantitative knowledge additional confirmed these findings (Determine S21a). The outcomes implied that Hb-loaded HMCN/Au mediated microwave-dynamic impact appeared to facilitate antigen presentation to DCs and set off downstream immune response. Given to its vital function in selling DC maturation, the differentiation of T lymphocytes throughout the major tumor have been examined [48]. Notably, the larger CD8+ T cells infiltration have been evoked by microwave stimulus and HMCN/Au@Hb administration (Fig. 5b), and the upper intratumoral CD8+/CD3+ T cell ratios have been noticed in irradiated teams in contrast with non-irradiated teams, significantly together with HMCN/Au@Hb (Determine S21b). Subsequent, we carried out circulation cytometry evaluation in splenic homogenates of mice to analyze the activation of T cells in immune organs. The share of CD8+ T cells have been considerably elevated within the HMCN/Au@Hb + MW group in comparison with the opposite teams (Fig. 5c). Likewise, larger CD8+/CD3+ T cell ratio have been noticed within the HMCN/Au@Hb + MW group than that of the HMCN/Au + MW group (Determine S21c). These findings urged that the oxygen-amplified microwave-dynamic impact enabled infiltration of immune cells to a bigger extent.
Antitumor immune response. (a) Circulate cytometry of DC maturation in TDLNs with indicated remedies. (b) Circulate cytometry of T-cell activation in tumors and (c) spleens with indicated remedies. (d) ELISA evaluation of the TNF-α, IFN-γ, IL-6, and IL-12p70 ranges in serum and tumors. Immunofluorescence photos of (e) HIF-1α, (f) Pimonidazole and CD31+, (g) CD8, IFN-γ and Granzyme B publicity in tumors. Information have been all introduced as imply values ± SD (n = 3). (* represents p<0.05, ** represents p<0.01)
Furthermore, the function of varied cell-secreted pro-inflammatory elements, comparable to TNF-𝛼, IFN-𝛾, IL-6, and IL-12p70, all of which had an important perform in modulating immune responses, have been additional explored through ELISA assay [49]. ELISA assay portrayed an elevated serum secretion ranges of 1.28-, 1.68-, 1.61- and 1.33-fold for TNF-𝛼, IFN-𝛾, IL-6 and IL-12p70 in HMCN/Au@Hb + MW group than that within the HMCN/Au + MW group, respectively. As for intratumoral secretion ranges, HMCN/Au@Hb upon microwave irradiation yielded the best TNF-𝛼, IFN-𝛾, IL-6 and IL-12p70 secretions, whereas their respective secretions in different teams have been comparatively decrease, confirming that HMCN/Au@Hb plus MW may exert a strong activated-inflammatory impact (Fig. 5d).
Aiming to deepen our comprehension of the interplay between hypoxic aid and intrinsic antitumor immunity of HMCN/Au@Hb in vivo, immunofluorescence experiments have been then carried out on mouse tumor tissues. The precise hypoxic standing of tumors was evaluated by HIF-1α expression ranges [50]. Apparently, a major down-regulation was noticed within the HMCN/Au@Hb + MW group in comparison with the HMCN/Au + MW group (Fig. 5e, Determine S22). In the meantime, accumulating proof has proven that inadequate microwave therapy may irritate hypoxia and induce angiogenesis in tumor websites, pimodazole and CD31+ staining have been adopted for detecting hypoxic areas and microvessels [51]. In contrast with the MW group, a dramatically discount of inexperienced fluorescence (pimodazole staining) and crimson fluorescence (CD31+ staining) have been noticed within the HMCN/Au@Hb + MW group (Fig. 5f, Determine S22). It urged the improved oxygenation and fewer angiogenesis on the tumor website, which might probably contribute to reversing the immune-suppressive TME. Immunofluorescence evaluation for CD8+, IFN-γ and Granzyme B additional verified the elevated infiltration of effector cytotoxic CD8+ T lymphocytes in tumor websites [47]. CD8+, IFN-γ and Granzyme B infiltration together remedy teams have been larger than that of the monotherapy teams, and the HMCN/Au@Hb + MW group confirmed the strongest fluorescence intense (Fig. 5g, Determine S22). These findings confirmed the effectivity of HMCN/Au@Hb in changing naïve T cells into effector T cells (CD8+, IFN-γ and Granzyme B). Total, the above findings demonstrated that intrinsic oxygen-carrying capability of Hb induced a drastically enhanced microwave-dynamic impact. Altogether, HMCN/Au@Hb had the distinctive skill of in situ oxygen era and preserve in vivo hypoxia-relieving impact, which advantages for reversing the immune-suppressive microenvironment. This examine may pave the best way in the direction of expediting tumoricidal immunity.