Enrichment of the NF-κB Pathway in Each Tumor Epithelial and CAFs and Optimistic Correlation with the Illness Development
We built-in two single-cell RNA sequencing datasets of high-grade serous ovarian most cancers from the GEO database (GSE235329 and GSE184880). A complete of 80,850 cells have been clustered for additional bioinformatics evaluation after low-quality cells have been eliminated, normalized, dimensionality lowered, and built-in. We recognized 20,455 fibroblasts, which clustered into 5 subclusters, comprising one of many regular fibroblasts (NFs) and 4 of CAFs (Determine S1A, B). Initially, we in contrast genes between the NF and CAFs from the 4 clusters to discover their capabilities. PROGENy enrichment evaluation revealed vital enrichment of JAK-STAT, PI3K, VEGF, hypoxia signaling, NF-κB, TNFa, EGFR, and MAPK pathways for every CAF cluster (Determine S1C). Equally, we carried out PROGENy enrichment evaluation of differential genes between regular and malignant ovarian epithelium. VEGF, NF-κB, and TNFa pathways have been considerably enriched (Determine S1D). The NF-κB pathway performs a significant position within the TME, selling tumor cell development, influencing CAFs to control matrix reorganization and cytokine secretion, and regulating the expression of immunosuppressive molecules [31, 42]. Activation of the NF-κB pathway triggers tumors to generate downstream responses, reminiscent of apoptotic escape and TME reworking, resulting in tumor insensitivity to platinum-based chemotherapeutic brokers [43]. p65 performs a key position within the NF-κB pathway and is a serious transcription issue within the NF-κB pathway, regulating the transcription of a variety of genes. The EOC survival curves plotted utilizing the Kaplan–Meier plotter revealed a unfavorable correlation of excessive p65 expression with general survival (Determine S1E). Collectively, our findings recommend that the NF-κB pathway is considerably upregulated in tumor epithelial cells and CAFs and performs an necessary position in TME regulation, making it a possible therapeutic goal.
Characterization of PH20/CCM@PMCS
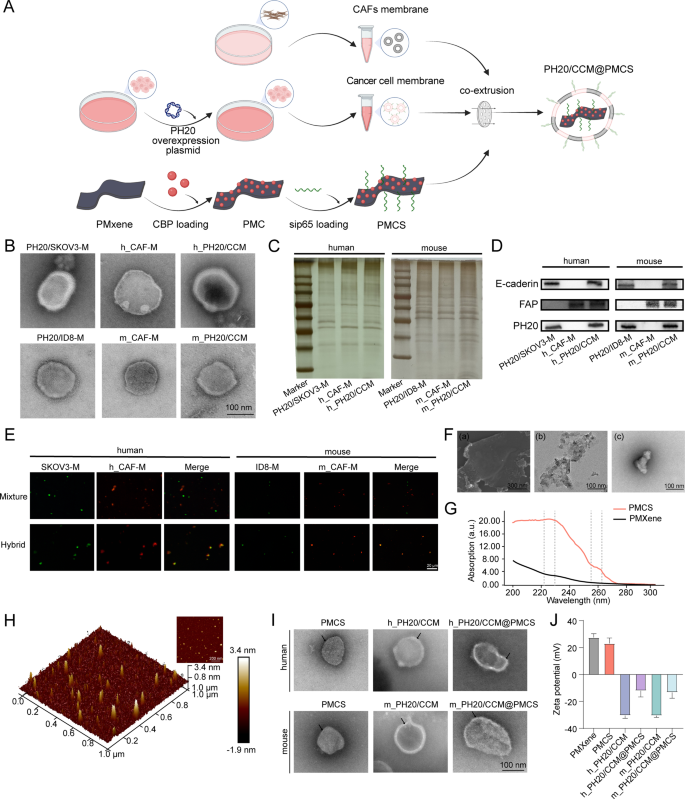
Determine 1A illustrates the preparation of PH20/CCM@PMCS within the following steps: (a) extraction of PH20-overexpressing tumor cell membranes and CAF membranes to type PH20/CCM; (b) MXene modification utilizing PDDA to acquire the PMXene and enriching CBP and siRNA on the floor of PMXene to assemble PMCS; (c) fusing PH20/CCM on the floor of PMCS by way of extrusion.
The PH20-overexpressing SKOV3 (PH20/SKOV3) membrane vesicles, human-derived CAF (h_CAF) membrane vesicles and human-derived PH20/CCM membrane vesicles (h_PH20/CCM) have been obtained by extruding the extracted PH20/SKOV3 membranes, h_CAF membranes, and PH20/SKOV3 and h_CAF membranes, respectively. Moreover, the PH20-overexpressing ID8 (PH20/ID8) membrane vesicles, mouse-derived CAF (m_CAF) membrane vesicles and mouse-derived PH20/CCM membrane vesicles (m_PH20/CCM) have been obtained by extruding the extracted PH20/ID8 membranes, m_CAF membranes, and PH20/ID8 and m_CAF membranes, respectively. Transmission electron microscopy (TEM) pictures revealed a darkish–brilliant–darkish cell membrane construction of the vesicles with 10–20 nm thickness (Fig. 1B), in keeping with a earlier research [44]. The cell membrane vesicles exhibited a unfavorable zeta potential (Determine S2), with no vital variations in look from totally different cell sources. We additional evaluated the protein options of the CAF membrane vesicles, PH20-overexpressing most cancers cell membrane vesicles, and PH20/CCM utilizing SDS–PAGE. The proteins on the hybrid membrane vesicles shared frequent traits with the PH20-overexpressing most cancers cell membrane vesicles and CAF membrane vesicles (Fig. 1C). Western blotting revealed the presence of particular proteins on the PH20-overexpressing most cancers cell membrane vesicles, CAF membrane vesicles, and PH20/CCM, together with E-cadherin, fibroblast activation protein alpha (FAP), and PH20 (Fig. 1D). This indicated that the functionalized membrane proteins have been retained within the membrane vesicles and co-expressed on the hybrid membrane vesicles after membrane hybridization. To confirm that the 2 cell membranes have been fused by co-extrusion and never easy mixing, the tumor cell membranes have been labeled with DIO (inexperienced), and the CAF membranes have been labeled with DID (crimson). The tumor cell (inexperienced) and CAF membranes (crimson) didn’t overlap within the mixing group, however overlapped within the co-extrusion group (Fig. 1E), indicating a profitable membrane hybridization after co-extrusion.
A excessive diploma of dispersibility and nanoscale planar measurement are important for MXene nanosheets to fulfill the stringent biomedical software necessities. MXene is proven in stacked blocks in scanning electron microscope (SEM) and TEM pictures (Fig. 1F(a) and 1F(b)), whereas additional sonication reveals extremely dispersed MXene in TEM pictures (Fig. 1F(c)). Subsequently, PMXene, PMXene@CBP (PMC), PMXene@sip65 (PMS), and PMCS have been efficiently synthesized. CBP and siRNA adhered to the floor of PMXene by electrostatic adsorption, and their loading effectivity and loading contents have been calculated (Determine S3). Contemplating the entrapment effectivity and drug loading contents, we finally selected to synthesize PMCS in a ratio of CBP: PMXene: siRNA = 30:10:0.25. Amongst them, the entrapment effectivity of CBP was 82.98%, the drug loading content material was 71.34%; the entrapment effectivity of siRNA was 71.79%, and the drug loading content material was 1.75%. The ultraviolet-visible absorption spectra have been used to research the principle elements of the PMCS. As proven in Fig. 1G, PMCS exhibited a powerful absorption peak close to 229 and 260 nm, similar to the binding of CBP and siRNA to PMXene, respectively. For drug launch (Determine S4), CBP initially confirmed explosive launch throughout the first 16 h and reached a plateau after 24 h. CBP exhibited the next launch charge at pH 5.0 than at pH 7.4, with roughly 70% drug launch. The discharge of siRNA additionally adopted this sample, with a drug launch charge of roughly 50% at pH 5.0. These findings indicated the wonderful efficiency of PMXene as a nanocarrier for loading and delivering CBP and siRNA. Pictures of the nanoparticles have been obtained utilizing an atomic drive microscope (AFM). The AFM picture revealed a layered construction of PMCS with a most diameter of roughly 100 nm and a thickness of 4–5 nm (Fig. 1H). PMCS was then encapsulated utilizing hybrid membrane vesicles. TEM pictures confirmed a layered morphology of PMCS with wonderful monodispersity. PH20/CCM@PMCS exhibited a core-shell construction with a darkish–brilliant–darkish cell membrane construction noticed on the floor, indicating the profitable modification utilizing the hybrid membrane (Fig. 1I). The general measurement distribution of PH20/CCM@PMCS detected utilizing dynamic mild scattering (DLS) was roughly 110 nm, indicating uniform particle measurement of PH20/CCM@PMCS (Determine S5). The zeta potentials of PMCS, m_PH20/CCM@PMCS, and h_PH20/CCM@PMCS have been (23.1 ± 4.1) mV, (− 12.1 ± 4.5) mV, and (− 13.3 ± 4.3) mV, respectively (Fig. 1J). The floor of the PH20/CCM@PMCS was negatively charged, which was helpful for the blood circulation time of the nanoparticles. After incubation with PBS, DMEM/F12, and DMEM/F12 with 10% FBS at 37 °C for twenty-four h, the diameter of the PH20/CCM@PMCS particles remained roughly 110 nm (Determine S6), indicating passable dispersibility and stability. After incubation of PH20/CCM@PMCS for 36 h, the cell membranes have been ruptured and nanoparticles have been quickly launched (Determine S7).
Characterization of hybrid membrane-coated nanoparticles. (A) The preparation process of PH20/CCM@PMCS. (B) TEM pictures of PH20/SKOV3 membrane vesicle (PH20/SKOV3-M), PH20/ID8 membrane vesicle (PH20/ID8-M), h_CAFs membranes (h_CAF-M), m_CAFs membranes (m_CAF-M), h_PH20/CCM, and m_PH20/CCM (Scale bar: 100 nm). (C) SDS-PAGE protein evaluation of most cancers cell membrane, cancer-associated fibroblast membrane, and hybrid cell membrane proteins. (D) Western Blot evaluation of attribute proteins from most cancers cell membrane, cancer-associated fibroblast membrane, and hybrid cell membrane proteins. (E) Pictures of blended membranes and hybrid membranes (Scale bar: 20 μm). (F) (a) SEM pictures of stacked block form MXene (Scale bar: 300 nm). (b) TEM pictures of stacked block form MXene (Scale bar: 100 nm) (c) TEM pictures of dispersed MXene (Scale bar: 100 nm) (G) UV-visible absorption spectra of PMXene and PMCS. (H) AFM picture of PMCS. (I) TEM pictures of PMXene, hybrid cell membranes and hybrid cell membranes coated PMXene (Scale bar: 100 nm). (J) The Zeta potential of PMXene, PMCS, h_PH20/CCM, h_PH20/CCM@PMCS, m_PH20/CCM, m_ PH20/CCM@PMCS. Knowledge are expressed as imply ± customary deviation (S.D.)
Operate of PH20/CCM@PMCS
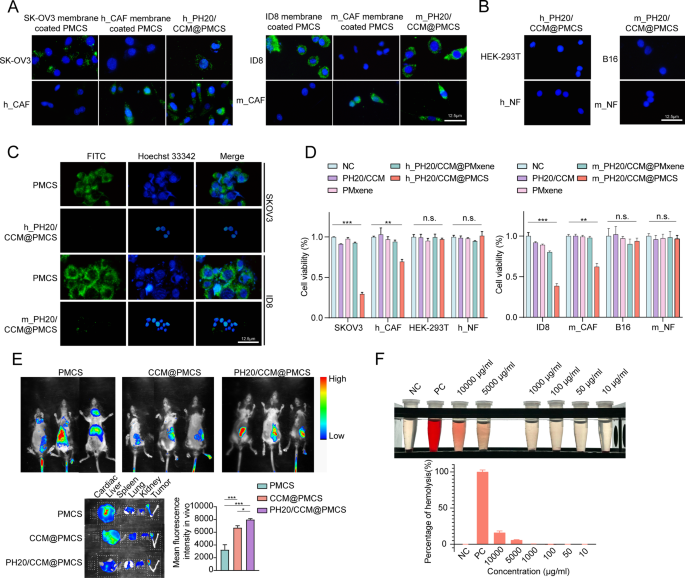
We validated the dual-targeting capability of PH20/CCM@PMCS towards most cancers cells and CAFs. Fluorescein isothiocyanate (FITC, inexperienced) was used to label PMXene. PMCS encapsulated by the SKOV3 cell membrane might be internalized by SKOV3 cells however not by h_CAFs. In distinction, PMCS encapsulated by h_CAF cell membranes might be internalized by h_CAFs, however not by SKOV3. SKOV3 and h_CAFs can concurrently encapsulate PMCS within the hybrid membrane. PMCS encapsulated by mouse-derived membranes additionally exhibited comparable properties (Fig. 2A). Particularly, the hybrid membrane exhibited a dual-targeting capability. As well as, PMCS encapsulated by h_PH20/CCM and m_PH20/CCM have been minimally internalized by different most cancers cells or NFs (Fig. 2B), indicating the excessive concentrating on impact of PH20/CCM@PMCS. Escape from the endothelial reticular system is important for all nanomedicines injected into the bloodstream. PMCS and PH20/CCM@PMCS have been subsequently co-cultured with RAW264.7 cells. The cell membrane shell successfully lowered the internalization of nanoparticles by macrophages (Fig. 2C). This can be attributed to the immune evasion perform of most cancers cell membranes [45]. To confirm the biocompatibility of PH20/CCM@PMCS in vitro, we carried out CCK8 experiments to look at the exercise of six cell sorts, SKOV3, ID8, h_CAFs, m_CAFs, 293T, and HUVECs, after co-incubation with PH20/CCM, PMXene, PH20/CCM@PMXene, and PH20/CCM@PMCS. Throughout co-incubation with most cancers cells, CAFs, 293T cells, and HUVECs, PH20/CCM, PMXene, and PH20/CCM@PMXene didn’t inhibit cell viability, indicating that they aren’t considerably poisonous. PH20/CCM@PMCS inhibited the viability of most cancers cells and CAFs, however had no vital impact on 293T cells or HUVECs (Fig. 2D). The results of various concentrations of PH20/CCM@PMCS on most cancers cells and CAFs are demonstrated in Determine S8. The above outcomes reveal that the cell membranes and nanocarrier PMXene themselves don’t have inhibitory results on cells. PH20/CCM@PMXene solely exerts inhibitory results on its goal cells, indicating the great biosafety of PH20/CCM@PMXene.
The concentrating on and penetrating talents of PH20/CCM@PMCS have been evaluated in vivo. PMXene was labeled with CY7 and encapsulated in PH20/CCM earlier than injection into orthotopic tumor-bearing C57BL/6 mice by the tail vein. Utilizing an in vivo fluorescence imaging system (Fig. 2E), vital aggregation of CCM@PMCS and PH20/CCM@PMCS on the tumor website was noticed in mice 24 h after intravenous injection, which was not noticed within the PMCS group. PH20/CCM@PMCS was extra concentrated throughout the tumor tissue than CCM@PMCS, indicating that PH20/CCM@PMCS might successfully penetrate the bodily barrier within the tumor tissue, attaining deep therapy. Neither the guts nor the spleen confirmed vital fluorescence accumulation; nevertheless, within the CCM@PMCS and PH20/CCM@PMCS teams, the liver, lungs, and kidneys exhibited slight fluorescence after 24 h following intravenous injection, indicating the tumor-targeting capability of cell membrane-encapsulated nanoparticles.
A crimson blood cell hemolysis assay was used to judge the blood compatibility of PH20/CCM@PMCS at totally different concentrations (PMXene focus: 10–10,000 µg mL-1). The proportion of hemolysis was calculated based mostly on the absorbance of the supernatant of the PH20/CCM@PMCS and the crimson blood cell resolution combination (Fig. 2F). Beneath the working focus (10 µg mL-1), negligible hemolysis (< 5%) was noticed, confirming the great blood compatibility of the nanoparticles.
Twin-targeting, penetrating functionality, and biocompatibility of PH20/CCM@PMCS. (A) Pictures of SKOV3 and h_CAF handled with SKOV3 membrane-coated PMCS, h_CAFs membrane-coated PMCS, and h_PH20/CCM-coated PMCS for 7 h. Pictures of ID8 and m_CAF handled with ID8 membrane-coated PMCS, m_CAFs membrane-coated PMCS, and m_ PH20/CCM-coated PMCS for 7 h. The nucleus is stained with DAPI (blue), and PMCS is labeled with FITC (inexperienced) (Scale bar: 12.5 μm). (B) Pictures of HEK-293T and h_NF handled with h_ PH20/CCM@PMCS for 7 h. Pictures of B16 and m_NF handled with m_PH20/CCM@PMCS for 7 h (Scale bar: 12.5 μm). (C) Pictures of RAW264.7 handled with PMCS, h_PH20/CCM@PMCS, and m_PH20/CCM@PMCS for 7 h (Scale bar: 12.5 μm). (D) CCK-8 assay indicated PH20/CCM, PMxene, and PH20/mPMxene had no vital inhibitory impact on both most cancers cells or regular cells (N = 3). (E) The penetrating capability of PH20/CCM@PMCS was investigated by the orthotopic homograft in vivo (N = 3). The fluorescence pictures of harvested main organs and tumor at 24 h post-injection. (F) Proportion of crimson blood cell hemolysis at totally different concentrations of PH20/CCM@PMCS (N = 3). Knowledge are expressed as imply ± S.D. and analyzed by bizarre one-way ANOVA. (* P < 0.05, ** P < 0.01, *** P < 0.001, n.s. no significance)
Impact of PH20/CCM@PMCS on ovarian tumors in Vitro
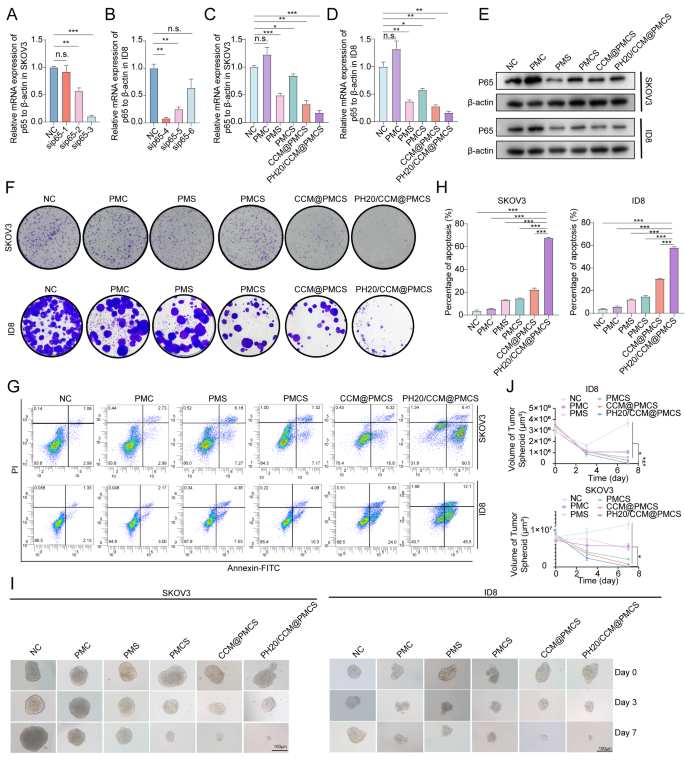
To pick the simplest siRNA concentrating on p65 in human cells, we verified three siRNAs (sip65 #1, sip65 #2, and sip65 #3) concentrating on human p65 RNA in SKOV3 cells (Fig. 3A). One other three siRNAs concentrating on p65 in mouse cells (sip65 #4, sip65 #5, and sip65 #6) have been assessed in ID8 for knockdown effectivity (Fig. 3B). The 2 siRNA strands with the very best knockdown effectivity (sip65 #3 and sip65 #4) have been chosen to assemble the nanoparticles. Subsequently, we evaluated the mRNA and protein expression of p65 within the cells handled with PMC, PMS, PMCS, CCM@PMCS, and PH20/CCM@PMCS. The RNA and protein ranges of p65 decreased considerably following PMS therapy, indicating that the nanoparticles efficiently launched siRNA into the cells and knocked down the goal RNA. The RNA and protein ranges of p65 elevated following PMC therapy, however these within the PMCS, CCM@PMCS, and PH20/CCM@PMCS teams handled with sip65 have been decrease than these within the NC group (Fig. 3C, D, E). Subsequent, we explored the antitumor efficacy of PH20/CCM@PMCSs in vitro. The cloning formation assay confirmed that PH20/CCM@PMCS considerably inhibited ovarian most cancers cell development (Fig. 3F). As well as, in contrast with that within the NC and PMCS teams, a big enhance in apoptosis was noticed within the tumor cells handled with PH20/CCM@PMCS (Fig. 3G, H). We additional investigated the antitumor efficacy of the engineered cell membrane-coated nanoparticles PH20/CCM@PMCS underneath advanced 3D tradition circumstances by incubating PH20/CCM@PMCS with 3D cultured tumorspheres. A comparability of the modifications within the quantity of the tumorspheres after 7 days revealed that the amount of the tumorspheres decreased most after therapy with PH20/CCM@PMCS (Fig. 3I, J). This can be attributed to the stronger penetration of the encapsulation membrane after the engineered PH20 modification, indicating excessive efficacy of PH20/CCM@PMCS in antitumor remedy in vitro.
Anti-tumor impact of PH20/CCM@PMCS in vitro. (A) Knockdown effectivity of sip65 in SKOV3 (N = 3). (B) Knockdown effectivity of sip65 in ID8 (N = 3). (C) Relative RNA expression of p65 to β-actin in SKOV3 in every group (N = 3). (D) Relative RNA expression of p65 to β-actin in ID8 in every group (N = 3). (E) Relative protein expression of p65 in SKOV3 and ID8 in every group. (F) Colony formation assay pictures of tumor cells handled with PMC, PMS, PMCS, CCM@PMCS, PH20/CCM@PMCS. (G, H) Detection and quantitative evaluation of apoptosis in SKOV3 and ID8 handled in every group by circulation cytometry (N = 3). (I, J) 3D development of tumors picture and quantitative evaluation of tumor sphere in NC, PMC, PMS, PMCS, CCM/PMCS, and PH20/CCM@PMCS group (Scale bar: 100 μm, N = 3). Knowledge are expressed as imply ± S.D. and analyzed by bizarre one-way ANOVA. (* P < 0.05, ** P < 0.01, *** P < 0.001, n.s. no significance)
PH20/CCM@PMCS Induced Immunogenic Cell Demise in Tumor cells
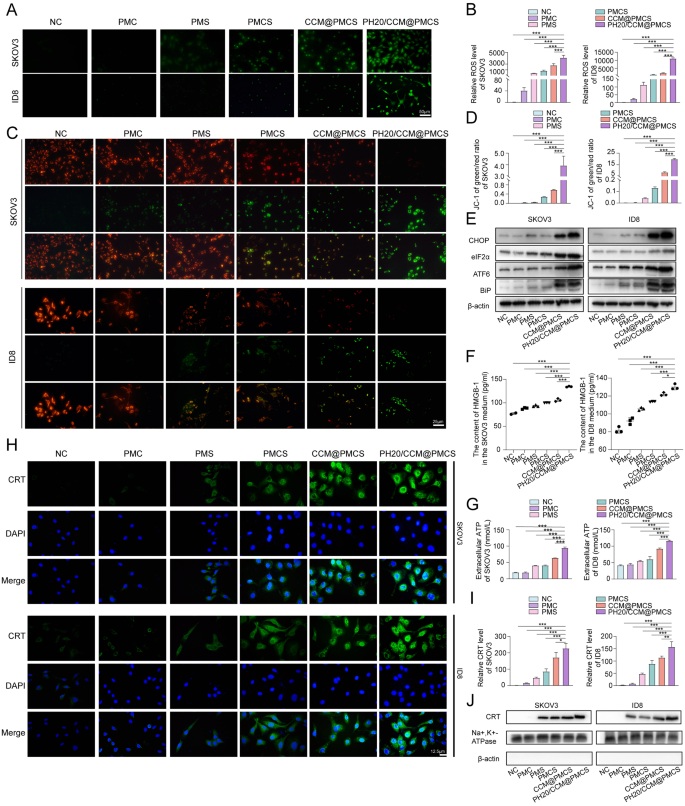
Contemplating that NF-κB is a vital regulatory pathway for reactive oxygen species (ROS) manufacturing in tissue cells, the promotion of cell apoptosis by PH20/CCM@PMCS might rely upon elevated intracellular oxidative stress [46]. The technology of ROS was detected by way of DCFH-DA probes (inexperienced). The PMC group exhibited few ROS, whereas the PH20/CCM@PMCS group exhibited an intense enhance in ROS ranges (Fig. 4A, B). Furthermore, the mitochondrial membrane potential was assessed utilizing the JC-1 probe. After PH20/CCM@PMCS therapy, the mitochondrial membrane potential decreased (Fig. 4C, D), indicating a rise in mobile oxidative stress. Excessive intracellular ROS can lead to endoplasmic reticulum (ER) dysfunction [47, 48]. Due to this fact, we carried out protein blotting evaluation to analyze whether or not remedies of every group would have an effect on ER stress. The identification of ER stress-related proteins indicated that PH20/CCM@PMCS remarkably induced ER dysfunction in contrast with different examined teams (Fig. 4E). ER stress and ROS are necessary intracellular pathways that induce immunogenic cell demise (ICD) [49]. The translocation of calcium reticulum protein (CRT) to the cell membrane, extracellular launch of adenosine 5ʹ-triphosphate (ATP), and secretion of excessive mobility group protein cassette 1 (HMGB1) are basic options of ICD [50,51,52]. Due to this fact, we explored the above three indicators in every group to detect whether or not cells underwent ICD. We collected the cell tradition supernatant from the NC, PMC, PMS, PMCS, CCM@PMCS, and PH20/CCM@PMCS teams. The ELISA outcomes present that the supernatant of the PH20/CCM@PMCS group displays a big enhance within the degree of HMGB1 (Fig. 4F). Important ATP secretion was noticed within the PH20/CCM@PMCS group utilizing the luciferase assay (Fig. 4G). To confirm the membrane switch of calcium reticulum proteins, cell membrane immunofluorescence staining and western blot analyses have been carried out. The PH20/CCM@PMCS group confirmed a big enhance within the membrane switch of calcium reticulum protein (Fig. 4H, I,J). In brief, these outcomes reveal that PH20/CCM@PMCS successfully elevated intracellular ROS and led to ER stress to induce the manufacturing of damage-associated molecular patterns, thus triggering ICD in tumor cells.
PH20/CCM@PMCS induced immunogenic cell demise in tumor cells. (A, B) Fluorescence pictures with semiquantitative analyses of intracellular ROS manufacturing in SKOV3 and ID8 handled in every group (Scale bar: 50 μm). (C, D) Fluorescence pictures with semiquantitative analyses of SKOV3 and ID8 mitochondrial membrane potential (JC-1 probe) (Scale bar: 25 μm). (E) Ranges of CHOP, elF2α, ATF6, and BIP in SKOV3 and ID8 in every group. (F) The focus of HGBM1 launched by SKOV3 and ID8 handled in every group (N = 3). (G) The focus of ATP launched by SKOV3 and ID8 handled in every group (N = 3). (H, I) Fluorescence pictures with semiquantitative analyses of CRT expression on the cell membrane of SKOV3 and ID8 (Scale bar: 12.5 μm). (J) Protein expression of CRT on the cell membrane of SKOV3 and ID8. Na+, Okay+-ATPase serves as an inner reference for cell membrane proteins. β-actin, a cytoplasmic protein was not noticed to reveal cytoplasmic elimination. Knowledge are expressed as imply ± S.D. and analyzed by bizarre one-way ANOVA. (* P < 0.05, ** P < 0.01, *** P < 0.001)
PH20/CCM@PMCS regulate cytokine secretion in CAFs to reverse tumor-promoting TME
CAFs foster a supportive atmosphere for tumor development [53, 54]. Moreover, CAFs promote tumor formation and development by producing numerous cytokines, development elements, and ECM proteins, which contribute to blood and lymphatic vessel formation, ECM reworking, immune system suppression, drug resistance, and tumor invasion [55,56,57,58]. Therefore, suppressing the discharge of cancer-promoting substances from CAFs would possibly alter the TME, making it helpful for inhibiting tumor development. To additional examine the impact of PH20/CCM@PMCS on CAFs, the cytokine ranges within the CAF supernatants handled with PH20/CCM@PMCS have been measured utilizing the Cytokine Proteome Profiler Array (Fig. 5A, B). Amongst them, 8 cytokines have been downregulated, and 15 have been upregulated. KOBAS3.0 was used for pathway enrichment evaluation of the differential cytokines, and vital enrichment in angiogenesis, inflammatory immunity, and cell migration and adhesion was noticed (Fig. 5C). The 23 differentially expressed cytokines have been categorized into three teams based mostly on their capabilities and the outcomes of KEGG enrichment evaluation (Fig. 5D), and their capabilities are listed in Desk S1. The highest six differentially expressed cytokines included GDF-15, GROα, Dkk-1, MMP-9, CD14, and osteopontin. Amongst them, osteopontin, Dkk-1, and MMP-9 take part in ECM reworking and tumor cell invasive migration. Particularly, osteopontin induces HIF-1α to advertise ovarian most cancers development and metastasis by activating the PI3-Okay/Akt survival pathway [59]. The DKK-1 protein inhibits tumor cell survival and migration by regulating β-catenin/E-cadherin signaling [60]. MMP-9 permits migration and invasion of ovarian most cancers cells by degrading basement membrane elements, finally resulting in metastasis [61]. CD14, DPPIV, and GDF-15 are concerned in inflammatory cell recruitment and immune cell infiltration and activation. Particularly, CD14excessive CD16low monocytes induce M2-like tumor-associated macrophages phenotype and suppress dendritic cell-activated CD4 + T-cell responses in ovarian most cancers [62]. DPPIV is concerned within the signal-transducing course of. Cross-linking of DPPIV and CD3 with immobilized mAbs can induce T-cell activation and IL-2 manufacturing [63]. GDF15 promotes the manufacturing of inducible Treg cells of peripheral origin in hepatocellular carcinoma by interacting with CD48 [64]. Furthermore, platinum-based chemotherapy has been reported to extend the degrees of circulating GDF-15 in sufferers with most cancers [65, 66]. Moreover, cytokines that play a key position in angiogenesis—angiogenin and VEGF—have been notably lowered. In conclusion, modifications in cytokines secreted by CAFs associated to invasion, angiogenesis, and immune infiltration have been noticed following therapy with PH20/CCM@PMCS, and their actual results on TME required verification by additional experiments.
PH20/CCM@PMCS alters cytokine ranges secreted by CAFs. (A, B) Cytokine Proteome Profiler Array pictures and semiquantitative evaluation of the supernatant of h-CAF handled with PH20/CCM@PMCS. (C) Pathway enrichment evaluation of differentially secreted cytokines. (D) Schematic diagram of the consequences of PH20/CCM@PMCS on CAF-mediated TME reworking from three features: inhibition of angiogenesis, suppression of most cancers cell invasion, and activation of immunity. Knowledge are expressed as imply ± S.D
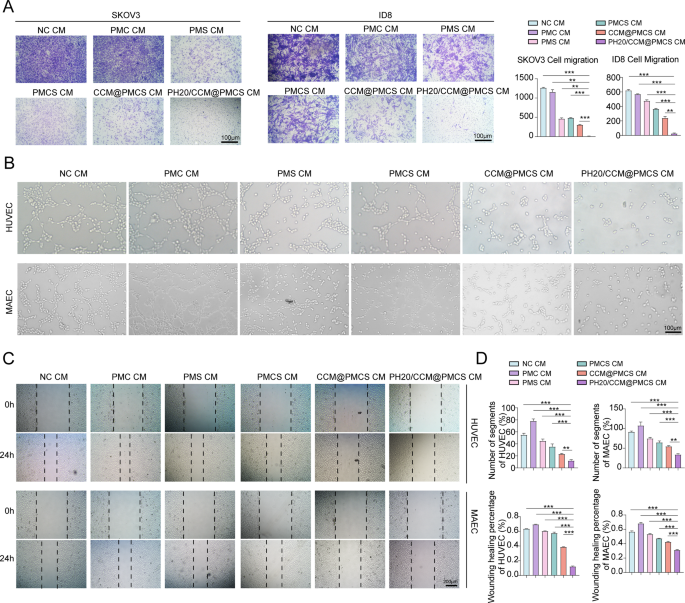
Subsequent, we co-cultured most cancers cells with conditioned medium (CM). The invasion functionality of tumor cells was investigated utilizing Transwell assay (Fig. 6A). In contrast with tumor cells incubated with CM from different teams, a big discount within the migration charge was noticed within the cells handled with PH20/CCM@PMCS CM. Thereafter, we explored the anti-angiogenic traits of CAFs handled in every group. The CM from the PH20/CCM@PMCS group hindered the migration and tube formation talents of vascular endothelial cells in vitro in contrast with these from different teams (Fig. 6B, C,D).
PH20/CCM@PMCS remodels TME by influencing cytokine secretion of CAFs. (A) Pictures of transwell assay and the relative migratory variety of ovarian most cancers cells handled with NC CM, PMC CM, PMS CM, PMCS CM, CCM@PMCS CM, and PH20/CCM@PMCS (Scale bar: 100 μm, N = 3). (B) Tube formation assays of HUVEC handled with totally different CMs (Scale bar: 100 μm). (C) Wound therapeutic assays of HUVEC handled with totally different CMs (Scale bar: 200 μm). (D) The statistical outcomes of tube formation assays and wound therapeutic assays (N = 3). Knowledge are expressed as imply ± S.D. and analyzed by bizarre one-way ANOVA. (* P < 0.05, ** P < 0.05, *** P < 0.001)
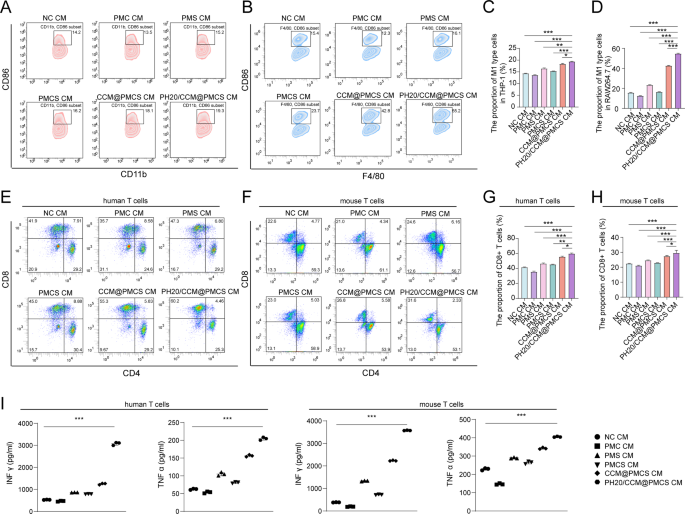
Subsequently, we investigated the consequences of CAFs on immune perform after PH20/CCM@PMCS therapy. In contrast with NC, the CM from h_PH20/CCM@PMCS elevated the typical share of CD86 + CD11b + macrophages from 14.2 to 19.3%; whereas, the CM from m_PH20/CCM@PMCS elevated the typical share of CD86 + F4/80 + macrophages from 15.4 to 55.2% (Fig. 7A, B,C, D). This can be attributed to the inhibition of the NF-κB pathway, which is concerned within the tumor inflammatory response. The cytokines secreted by CAFs handled with PH20/CCM@PMCS considerably stimulated macrophage polarization towards the M1 kind in comparison with the PMCS therapy. This impact might probably happen as a result of elevated internalization of nanoparticles by CAFs and the improved internalization of hybrid-cell membranes by macrophages as tumor antigens, additional selling their polarization towards the M1 kind. These outcomes indicated that PH20/CCM@PMCS might contribute to macrophage polarization and reshape the TME. CD8 + cytotoxic T cells (CTL) are one of many key immune surveillance cells within the TME, that are a constructive indicator of prognosis. Growing the proportion of CTL within the tumor tissues of the sufferers may also help inhibit tumor development. To check the impact of CAFs on CTL activation following PH20/CCM@PMCS therapy, we cultured T cells utilizing CMs from totally different therapy teams. The variety of activated T cells that differentiated into CD8 + T cells within the PH20/CCM@PMCS group was nearly 50% larger than that within the NC group (Fig. 7E, F,G, H). Additional, we assessed IFN-γ and TNF-α secreted from T cells in every therapy group by ELISA. The best IFN-γ and TNF-α ranges have been noticed within the PH20/CCM@PMCS group, indicating useful activation of CTL (Fig. 7I). Collectively, PH20/CCM@PMCS regulates cytokine secretion in CAFs to reverse the tumor-promoting TME.
PH20/CCM@PMCS remodels the immune atmosphere by influencing cytokine secretion of CAFs. (A) Proportion of M1 macrophages (CD86 + CD11b+) in THP-1 after therapy with totally different CMs (N = 3). (B) Proportion of M1 macrophages (CD86 + F4/80+) in RAW264.7 after therapy with totally different CMs. (C) The statistical outcomes of M1 kind THP-1 proportion. (D) The statistical outcomes of M1 kind RAW264.7 proportion. (E) Proportion of human CD8 + T cells after therapy with totally different CMs (N = 3). (F) Proportion of mouse CD8 + T cells after therapy with totally different CMs (N = 3). (G) The statistical outcomes of human CD8 + T cell proportion. (H) The statistical outcomes of mouse CD8 + T cell proportion. (I) IFN γ and TNF α have been quantified by ELISA, within the tradition supernatants of T cells stimulated with totally different teams (N = 3). Knowledge are expressed as imply ± S.D. and analyzed by bizarre one-way ANOVA. (* P < 0.05, ** P < 0.05, *** P < 0.001)
Inhibitory impact of PH20/CCM@PMCS in orthotopic homograft
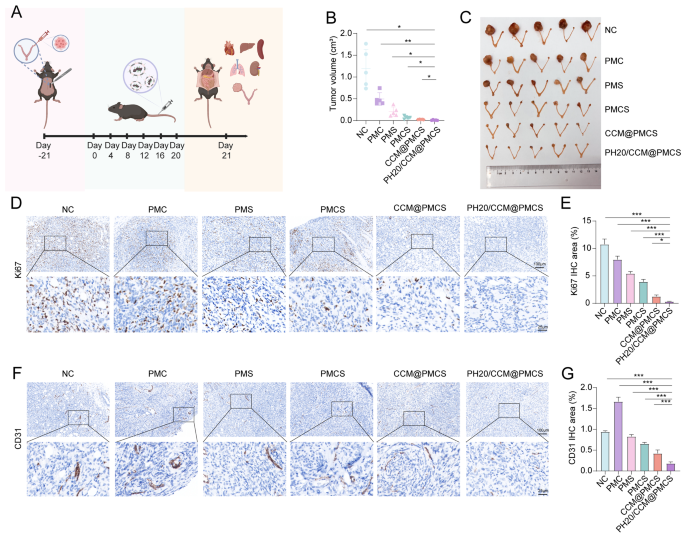
To substantiate the position of PH20/CCM@PMCS in EOC development in vivo, we chosen wholesome C57BL/6 mice to assemble orthotopic homografts and designed in vivo experiments (Fig. 8A). Tumor-bearing mice have been randomly divided into six teams and injected with physiological saline, PMC, PMS, PMCS, CCM@PMCS, or PH20/CCM@PMCS by the tail vein on Day 0, 4, 8, 12, 16, and 20. In the course of the therapy interval, no vital variations in physique weight have been noticed amongst totally different teams of mice (Determine S9). After therapy, we collected the tumors from every group and measured their volumes. Remedy of PMC and PMS confirmed a slight inhibitory impact on tumors in contrast with the NC group. In distinction, tumor development was considerably inhibited within the PH20/CCM@PMCS group. In contrast with the tumor quantity of the PMCS group with out hybrid membrane protection, the tumor quantity of the CCM@PMCS and PH20/CCM@PMCS teams with hybrid membrane protection was lowered by 63.37% and 85.30%, respectively. This implies a stronger anti-tumor impact of hybrid membrane-coated nanoparticles, which can be associated to the larger quantities of hybrid membrane-coated nanoparticles being ingested. Moreover, the robust penetration of PH20/CCM@PMCS will increase the supply of nanoparticles, making the efficacy much more vital. In conclusion, PH20/CCM@PMCS considerably reversed the expansion of the homograft tumors (Fig. 8B, C). In contrast with different teams, the variety of Ki67-positive cells stained by way of immunohistochemistry (IHC) was considerably lowered after therapy with PH20/CCM@PMCS or CCM@PMCS (Fig. 8D, E), indicating a stronger inhibition of tumor proliferation. The tumor tissue was stained utilizing Masson’s staining to look at its histopathology. Notably, within the PH20/CCM@PMCS therapy group, the tumor tissue was sparse, and the cell nucleus measurement decreased, whereas within the management group, the tumor tissue was dense, and the cell nucleus measurement confirmed robust heterogeneity (Determine S10).
Moreover, we investigated the antitumor mechanisms of those nanoparticles. We evaluated the formation of blood vessels in tumor tissues by measuring CD31 expression. Apparently, PMC promoted tumor angiogenesis, which was just like a earlier scientific research and could also be related to chemotherapeutic drug-induced hypoxia [67]. In contrast with NC, the CD31 ranges within the PMS and PMCS teams didn’t change considerably, whereas the CD31 ranges within the PH20/CCM@PMCS group went down almost 75%, suggesting that PH20/CCM@PMCS had an inhibitory impact on tumor angiogenesis (Fig. 8F, G).
Anti-tumor impact of PH20/CCM@PMCS in orthotopic homograft. (A) Experimental process in vivo. (B) Tumor quantity after 21-day therapy (N = 5). (C) Excised ovaries and uterus from NC, PMC, PMS, PMCS, CCM@PMCS, PH20/CCM@PMCS after 21-day therapy. (D, E) Immunohistochemical staining of Ki67 with semiquantitative analyses of excised tumor slices in every group (Scale bar: 10X: 100 μm, 40X: 25 μm, N = 3). (F, G) Immunohistochemical staining of CD31 with semiquantitative analyses of excised tumor slices in every group (Scale bar: 10X: 100 μm, 40X: 25 μm, N = 3). Knowledge are expressed as imply ± S.D. and analyzed by bizarre one-way ANOVA. (* P < 0.05, ** P < 0.05, *** P < 0.001)
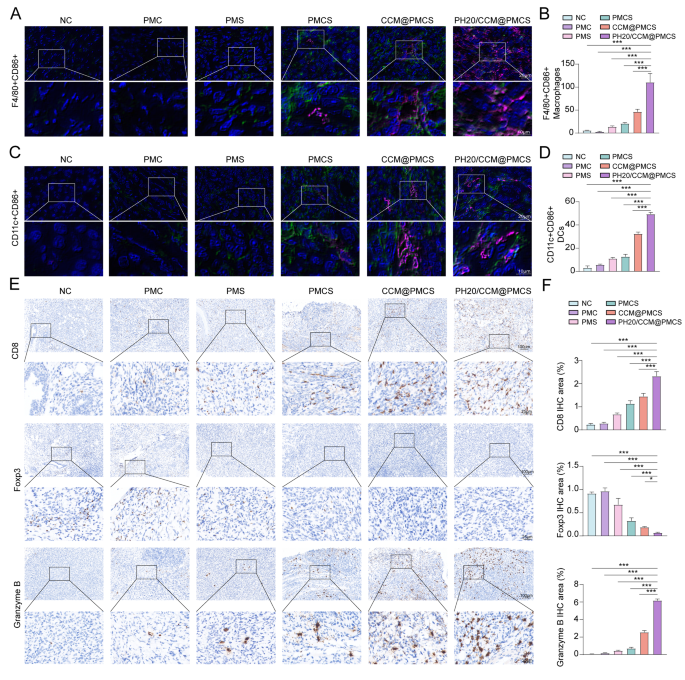
To confirm the impact of PH20/CCM@PMCS on immune activation in vivo, we measured the variety of macrophages within the tumor tissues of every mouse group. In contrast with different remedies, PH20/CCM@PMCS considerably elevated the aggregation of F4/80 (inexperienced) constructive macrophages within the tumor tissue, and the macrophages expressed CD86 (crimson), indicating polarization towards the M1 kind (Fig. 9A, B). To additional assess TME transformation, we measured the degrees of dendritic cell maturation on the tumor website. The degrees of CD11c+(inexperienced) CD86+(crimson) dendritic cells have been elevated within the PH20/CCM@PMCS group in contrast with the opposite teams (Fig. 9C, D), indicating that PH20/CCM@PMCS might contribute to dendritic cell maturation. Subsequently, we evaluated the T cells expressing CD8 and granzyme B within the tumor tissues utilizing IHC and noticed elevated cytotoxic T-cell aggregation within the PH20/CCM@PMCS-treated group. The staining of T cells with immunosuppressive perform (FOXP3-positive) revealed decreased Treg aggregation within the PH20/CCM@PMCS-treated group (Fig. 9E, F). These outcomes point out that PH20/CCM@PMCS relieved the TME by selling immune infiltration of the tumor tissue.
Results of PH20/CCM@PMCS on reworking immune infiltration in TME. (A, B) Immunofluorescence staining for F4/80 (inexperienced) and CD86 (crimson) with semiquantitative analyses of excised tumor slices in every group (Scale bar: 64X: 20 μm, X: 180X: 10 μm). (C, D) Immunofluorescence staining for CD11c (inexperienced) and CD86 (crimson) with semiquantitative analyses of excised tumor slices in every group (Scale bar: 64X: 20 μm, 180X: 10 μm). (E, F) Immunohistochemical staining of CD8, Foxp3, and Granzyme B with semiquantitative analyses of excised tumor slices in every group (Scale bar: 10X: 100 μm, 40X: 25 μm, N = 3). Knowledge are expressed as imply ± S.D. and analyzed by bizarre one-way ANOVA. (* P < 0.05, ** P < 0.05, *** P < 0.001)
Aside from these, various levels of most cancers lung metastasis have been noticed in mouse lungs. Hematoxylin and eosin (H&E)-stained pathological sections of metastatic lesions have been noticed (Determine S11), and in contrast with the NC group, each the PH20/CCM@PMCS and CCM@PMCS teams exhibited a big discount in metastatic lesions, indicating the inhibitory impact of PH20/CCM@PMCS on distant ovarian most cancers metastasis.
After 21 days of therapy, H&E staining of necessary organs of the mice, together with the guts, liver, spleen, lungs, and kidneys, revealed no vital harm to those organs (Determine S12A). The venous blood of the mice was collected for biochemical evaluation. Indicators such because the UA, CR, ALT, AST, albumin, and complete protein ranges have been all throughout the regular ranges (Determine S12B). The above outcomes demonstrated wonderful concentrating on and penetration talents and good biosafety of PH20/CCM@PMCS in mice in vivo.
Evaluation of steel nanodrug metabolism is important for additional in vivo software. To research the systemic circulation time of the nanoparticles, 10 µL of blood was collected for measurement at totally different time factors after injection into wholesome C57BL/6 mice. The plasma focus of the nanoparticles was negligible at 48 h after injection (Determine S13). Subsequent, we investigated the excretion and tissue deposition of PH20/CCM@PMCS in tumor-bearing mice. PH20/CCM@PMCS was injected intravenously into C57BL/6 mice loaded with orthotopic homografts. Ti ranges have been measured in urine and feces at 12, 24, and 48 h to observe excretion. Ti excretion within the urine and feces of mice after 48 h reached 20.09% and 16.80%, respectively (Determine S14). The guts, liver, spleen, lungs, and kidneys of mice have been collected 21 days after therapy to detect the biodistribution of nanoparticles. Intravenous PH20/CCM@PMCS was primarily deposited within the liver and spleen as a consequence of nanoparticle seize by the endothelial reticular system. Nanoparticles have been deposited within the kidneys, probably as a result of excretion of nanoparticles by the kidneys (Determine S15). Total, nanoparticle deposition in fundamental organs was low, just like the outcomes from in vivo fluorescence imaging in small animals. Excessive excretion and low off-target results of nanoparticles guarantee the protection of their scientific translation for most cancers remedy.
PH20/CCM@PMCS potentiates Anti-PD-L1 blockade remedy
PD-L1 monoclonal antibody is a extensively used immune checkpoint inhibitor for most cancers therapy, however its response charge is low in ovarian most cancers [68]. As PH20/CCM@PMCS induced ICD and enhanced immune infiltration within the TME, we evaluated its potential to enhance the efficacy of tumor immune checkpoint remedy. We administered PH20/CCM@PMCS individually, anti-PD-L1, or a mix of each in ID8 tumor-bearing mice (Determine S16A), whereas CBP was used as a typical therapy for management. Efficacy evaluation of ovarian in situ tumor development confirmed that anti-PD-L1 alone didn’t considerably have an effect on tumor development, suggesting that this tumor subtype exhibited a restricted response to immune checkpoint inhibition. In distinction, PH20/CCM@PMCS alone considerably inhibited tumor development and was simpler than CBP alone. Furthermore, tumor inhibition by PH20/CCM@PMCS was considerably augmented together with anti-PD-L1 (Determine S16B, C). The therapeutic interventions didn’t adversely have an effect on physiological parameters reminiscent of physique weight (Determine S16D) and liver and kidney perform (Determine S16E). Tumor development inhibition achieved utilizing PH20/CCM@PMCS was additional enhanced together with anti-PD-L1.