In an period the place information is the lifeblood of medical development, the medical trial {industry} finds itself at a essential crossroads. The present panorama of medical information administration is fraught with challenges that threaten to stifle innovation and delay life-saving therapies.
As we grapple with an unprecedented deluge of data—with a typical Part III trial now producing a staggering 3.6 million information factors, which is thrice greater than 15 years in the past, and greater than 4000 new trials licensed annually—our present information platforms are buckling beneath the pressure. These outdated techniques, characterised by information silos, poor integration, and overwhelming complexity, are failing researchers, sufferers, and the very progress of medical science. The urgency of this case is underscored by stark statistics: about 80% of medical trials face delays or untimely termination because of recruitment challenges, with 37% of analysis websites struggling to enroll satisfactory members.
These inefficiencies come at a steep price, with potential losses starting from $600,000 to $8 million every day a product’s improvement and launch is delayed. The medical trials market, projected to succeed in $886.5 billion by 2032 [1], calls for a brand new technology of Medical Information Repositories (CDR).
Reimagining Medical Information Repositories (CDR)
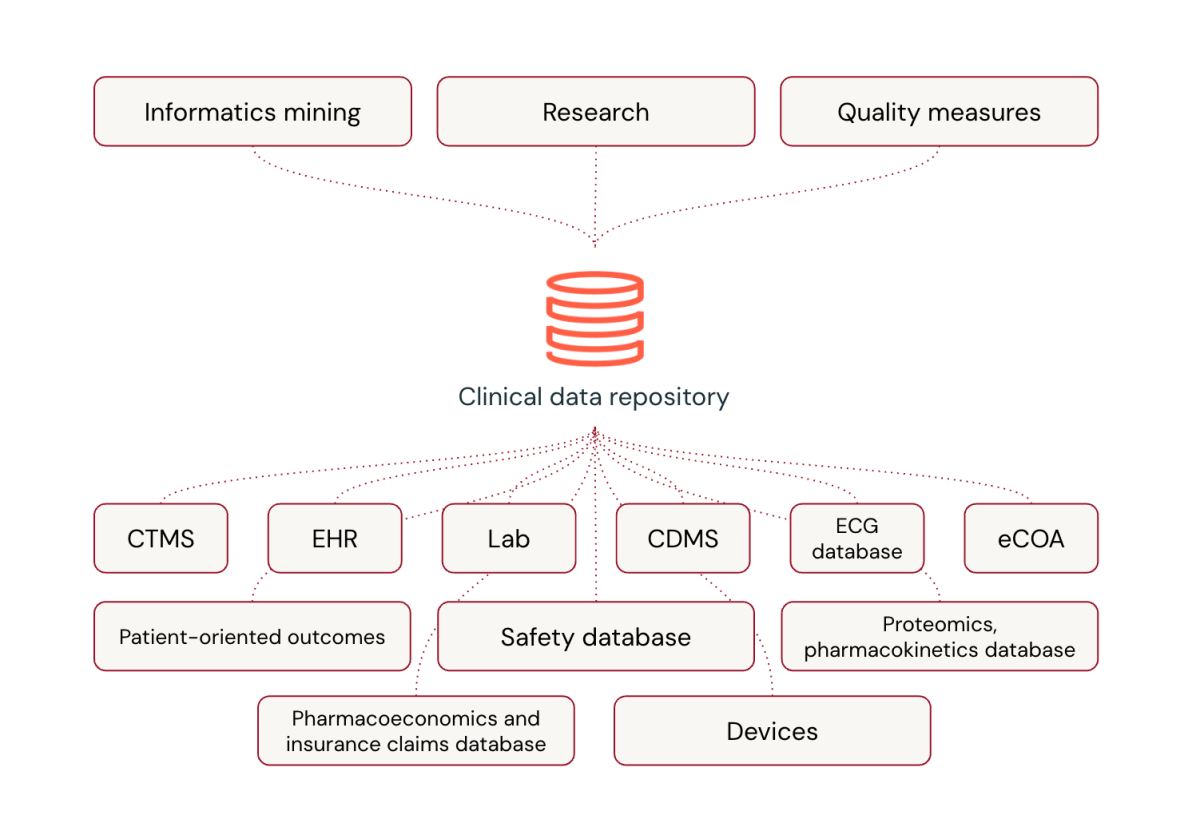
Sometimes, medical trial information administration depends on specialised platforms. There are numerous causes for this, ranging from the standardized authorities’ submission course of, the person’s familiarity with particular platforms and programming languages, and the power to depend on the platform vendor to ship area data for the {industry}.
With the worldwide harmonization of medical analysis and the introduction of regulatory-mandated digital submissions, it is important to know and function inside the framework of world medical improvement. This entails making use of requirements to develop and execute architectures, insurance policies, practices, pointers, and procedures to handle the medical information lifecycle successfully.
A few of these processes embody:
- Information Structure and Design: Information modeling for medical information repositories or warehouses
- Information Governance and Safety: Requirements, SOPs, and pointers administration along with entry management, archiving, privateness, and safety
- Information High quality and Metadata administration: Question administration, information integrity and high quality assurance, information integration, exterior information switch, together with metadata discovery, publishing, and standardization
- Information Warehousing, BI, and Database Administration: Instruments for information mining and ETL processes
These parts are essential for managing the complexities of medical information successfully.
Common platforms are remodeling medical information processing within the pharmaceutical {industry}. Whereas specialised software program has been the norm, common platforms supply important benefits, together with the flexibleness to include novel information varieties, close to real-time processing capabilities, integration of cutting-edge applied sciences like AI and machine studying, and sturdy information processing practices refined by dealing with large information volumes.
Regardless of considerations about customization and the transition from acquainted distributors, common platforms can outperform specialised options in medical trial information administration. Databricks, for instance, is revolutionizing how Life Sciences corporations deal with medical trial information by integrating numerous information varieties and offering a complete view of affected person well being.
In essence, common platforms like Databricks usually are not simply matching the capabilities of specialised platforms – they’re surpassing them, ushering in a brand new period of effectivity and innovation in medical trial information administration.
Leveraging the Databricks Information Intelligence Platform as a basis for CDR
The Databricks Information Intelligence Platform is constructed on high of lakehouse structure. Lakehouse structure is a contemporary information structure that mixes one of the best options of knowledge lakes and information warehouses. This corresponds properly to the wants of the fashionable CDR.
Though most medical trial information symbolize structured tabular information, new information modalities like imaging and wearable gadgets are gaining recognition. They’re the brand new approach of redefining the medical trials course of. Databricks is hosted on cloud infrastructure, which provides the flexibleness of utilizing cloud object storage to retailer medical information at scale. It permits storing all information varieties, controlling prices (older information could be moved to the colder tiers to avoid wasting prices however accommodate regulatory necessities of conserving information), and information availability and replication. On high of this, utilizing Databricks because the underlying know-how for CDR permits one to maneuver to the agile improvement mannequin the place new options could be added in managed releases in opposition to Large Bang software program model updates.
The Databricks Information Intelligence Platform is a full-scale information platform that brings information processing, orchestration, and AI performance to at least one place. It comes with many default information ingestion capabilities, together with native connectors and presumably implementing customized ones. It permits us to combine CDR with information sources and downstream purposes simply. This capability supplies flexibility and end-to-end information high quality and monitoring. Native assist of streaming permits to complement CDR with IoMT information and achieve close to real-time insights as quickly as information is out there. Platform observability is an enormous matter for CDR not solely due to strict regulatory necessities but additionally as a result of it permits secondary use of knowledge and the power to generate insights, which in the end can enhance the medical trial course of general. Processing medical information on Databricks permits for implementation of the versatile options to realize perception into the method. As an illustration, is processing MRI pictures extra resource-consuming than processing CT take a look at outcomes?
Implementing a Medical Information Repository: A Layered Method with Databricks
Medical Information Repositories are refined platforms that combine the storage and processing of medical information. Lakehouse medallion structure, a layered strategy to information processing, is especially well-suited for CDRs. This structure usually consists of three layers, every progressively refining information high quality:
- Bronze Layer: Uncooked information ingested from varied sources and protocols
- Silver Layer: Information conformed to plain codecs (e.g., SDTM) and validated
- Gold Layer: Aggregated and filtered information prepared for evaluation and statistical evaluation
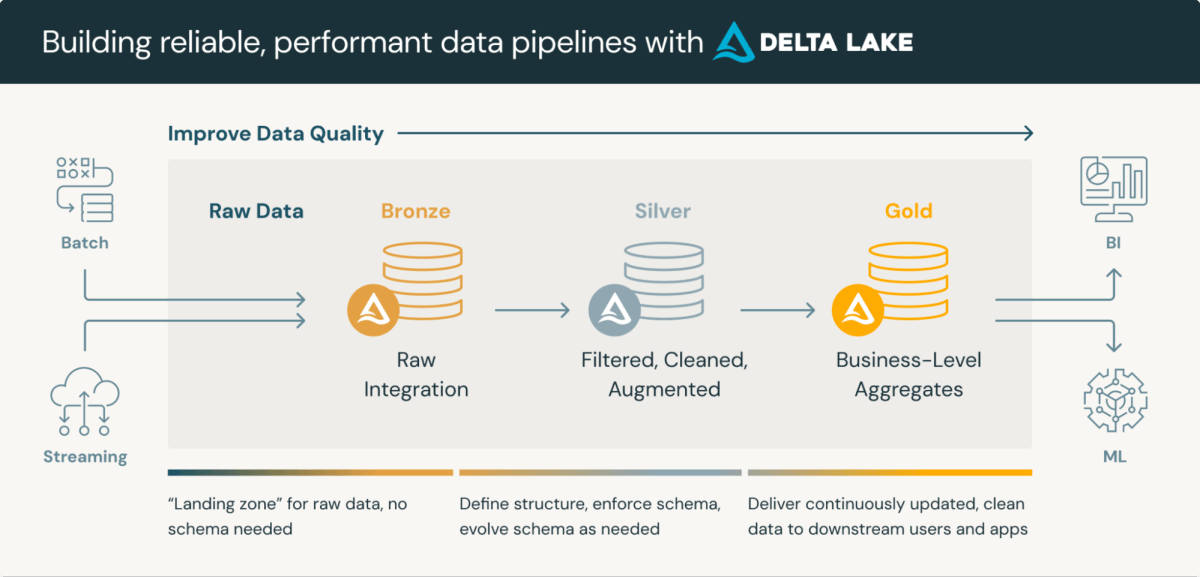
Using Delta Lake format for information storage in Databricks affords inherent advantages comparable to schema validation and time journey capabilities. Whereas these options want enhancement to totally meet regulatory necessities, they supply a stable basis for compliance and streamlined processing.
The Databricks Information Intelligence Platform comes outfitted with sturdy governance instruments. Unity Catalog, a key element, affords complete information governance, auditing, and entry management inside the platform. Within the context of CDRs, Unity Catalog permits:
- Monitoring of desk and column lineage
- Storing information historical past and alter logs
- Nice-grained entry management and audit trails
- Integration of lineage from exterior techniques
- Implementation of stringent permission frameworks to forestall unauthorized information entry
Past information processing, CDRs are essential for sustaining information of knowledge validation processes. Validation checks must be version-controlled in a code repository, permitting a number of variations to coexist and hyperlink to totally different research. Databricks helps Git repositories and established CI/CD practices, enabling the implementation of a sturdy validation test library.
This strategy to CDR implementation on Databricks ensures information integrity and compliance and supplies the flexibleness and scalability wanted for contemporary medical information administration.
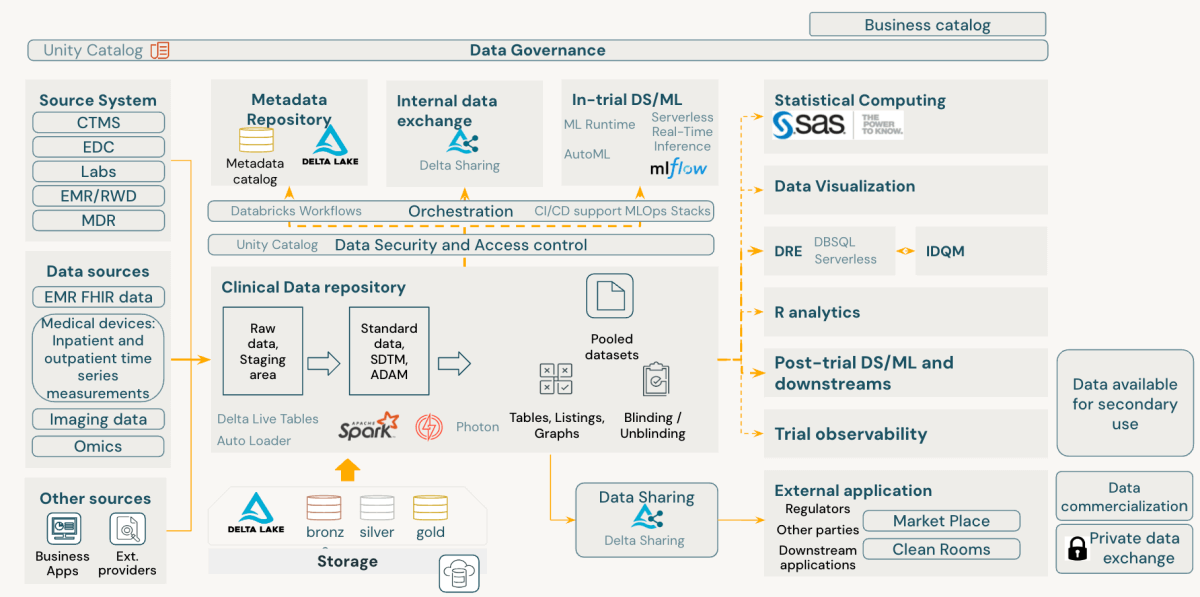
The Databricks Information Intelligence Platform inherently aligns with FAIR rules of scientific information administration, providing a complicated strategy to medical improvement information administration. It enhances information findability, accessibility, interoperability, and reusability whereas sustaining sturdy safety and compliance at its core.
Challenges in Implementing Trendy CDRs
No new strategy comes with out challenges. Medical information administration depends closely on SAS, whereas modem information platforms primarily make the most of Python, R, and SQL. This clearly introduces not solely technical disconnect but additionally extra sensible integration challenges. R is a bridge between two worlds — Databricks companions with Posit to ship first-class R expertise for R customers. On the identical time, integrating Databricks with SAS is feasible to assist migrations and transition. Databricks Assistant permits customers who’re much less acquainted with the actual language to get the assist required to jot down high-quality code and perceive the present code samples.
An information processing platform constructed on high of a common platform will all the time be behind in implementing domain-specific options. Sturdy collaboration with implementation companions helps mitigate this danger. Moreover, adopting a consumption-based value mannequin requires further consideration to prices, which have to be addressed to make sure the platform’s monitoring and observability, correct person coaching, and adherence to greatest practices.
The most important problem is the general success charge of all these implementations. Pharma corporations are continuously wanting into modernizing their medical trial information platforms. It’s an interesting space to work on to shorten the medical trial period or discontinue trials that aren’t more likely to grow to be profitable quicker. The quantity of knowledge collected now by the common pharma firm incorporates an unlimited quantity of insights which are solely ready to be mentioned. On the identical time, the vast majority of such tasks fail. Though there is no such thing as a silver bullet recipe to make sure a 100% success charge, adopting a common platform like Databricks permits implementing CDR as a skinny layer on high of the present platform, eradicating the ache of frequent information and infrastructure points.
What’s subsequent?
Each CDR implementation begins with the stock of the necessities. Though the {industry} follows strict requirements for each information fashions and information processing, understanding the boundaries of CDR in each group is important to make sure venture success. Databricks Information Intelligence Platform can open many extra capabilities to CDR; that’s why understanding the way it works and what it affords is required. Begin with exploring Databricks Information Intelligence Platform. Unified governance with Unity Catalog, information ingestion pipelines with Lakeflow, information intelligence suite with AI/BI and AI capabilities with Mosaic AI shouldn’t be unknown phrases to implement a profitable and future-proof CDR. Moreover, integration with Posit and superior information observability functionally ought to open up the potential of taking a look at CDR as a core of the Medical information ecosystem relatively than simply one other a part of the general medical information processing pipeline.
Increasingly more corporations are already modernizing their medical information platforms by using fashionable architectures like Lakehouse. However the huge change is but to return. The enlargement of Generative AI and different AI applied sciences is already revolutionizing different industries, whereas the pharma {industry} is lagging behind due to regulatory restrictions, excessive danger, and value for the mistaken outcomes. Platforms like Databricks enable cross-industry innovation and data-driven improvement to medical trials and create a brand new mind-set about medical trials typically.
Get began in the present day with Databricks.
Quotation:
[1] Medical Trials Statistics 2024 By Phases, Definition, and Interventions
[2] Lu, Z., & Su, J. (2010). Medical information administration: Present standing, challenges, and future instructions from {industry} views. Open Entry Journal of Medical Trials, 2, 93–105. https://doi.org/10.2147/OAJCT.S8172
Be taught extra concerning the Databricks Information Intelligence Platform for Healthcare and Life Sciences.

