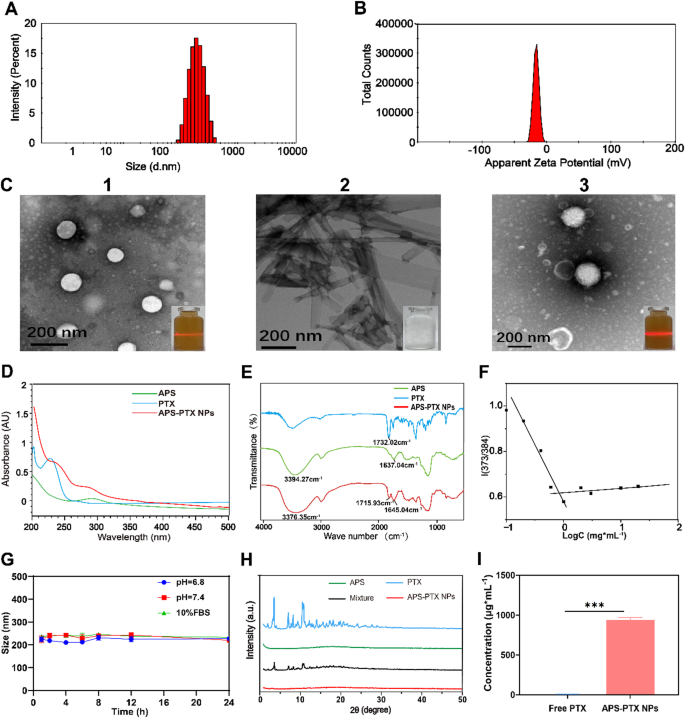
Characterization of APS-PTX NPs. (A) Dimension distribution of APS-PTX NPs; (B) Zeta potential of APS-PTX NPs; (C) TEM photos of APS(1), free PTX(2) and APS-PTX NPs(3); (D) UV-vis spectrum of APS, free PTX and APS-PTX NPs; (E) FT-IR spectrum of APS, free PTX and APS-PTX NPs; (F) CMC values of APS; (G) Stability of APS-PTX NPs in pH 6.8, 7.4 PBS and 10% FBS for twenty-four h (n = 3); (H) XRD spectrum of APS-PTX NPs, their monomers and their mixtures; (I) Solubility of PTX
Preparation and characterization of APS-PTX NPs
APS-PTX NPs have been ready by a easy one-step sonication methodology. After course of optimization (The optimization course of is proven in Desk S2-S4), the typical particle measurement of APS-PTX NPs was 191.7 ± 4.6 nm (Fig. 2A), with a polydispersity index (PDI) of 0.15 ± 0.03 and a zeta potential of −18.34 ± 0.43 mV (Fig. 2B). Transmission electron microscopy (TEM) photos confirmed that APS itself may type white spherical self-assembled nanoparticles in aqueous resolution, accompanied by the Tyndall impact (Fig. 2C1); free PTX had a black prismatic crystal construction and precipitated in aqueous resolution (Fig. 2C2), whereas APS-PTX NPs consisted of black spherical nanoparticles because the core and white materials because the shell, indicating that APS-PTX NPs is fashioned by APS encapsulation of PTX, and exhibited a extra apparent Tyndall impact than the APS aqueous resolution (Fig. 2C3). CMC is likely one of the essential parameters to measure the flexibility of amphiphilic polymers to type nanoparticles [43] and subsequently it’s crucial to find out the CMC worth of APS. On this examine, as proven in Fig. 2F two straight strains have been obtained (Y1 = 0.56401-0.53398x; Y2 = 0.61841 + 0.01894x), and the intersection level was calculated by means of Y1 and Y2 to get the CMC. The CMC of APS was 0.79 mg/mL, which meant APS had micelle-like properties. This means that after easy co-sonication, APS can encapsulate the hydrophobic chemotherapeutic drug to type new nanoparticles. Curiously, APS exhibited a solubilization perform much like that of Liposomes. The solubility of free PTX in aqueous resolution was 8.78 ± 0.76 µg·mL−1, whereas the focus of PTX in APS-PTX NPs was 941.96 ± 29.28 µg·mL−1, with a 107-fold enhance in solubility (Determine I). The encapsulation effectivity (EE) and the drug loading (DL) of PTX in APS-PTX NPs have been 99.47 ± 0.18% and 9.66 ± 0.12%. UV–seen spectrophotometry of APS-PTX NPs and free APS and PTX on the identical focus was carried out. As proven in Fig. 2D, free PTX exhibited most absorption peaks at 230 nm. In distinction, the absorption depth of APS-PTX NPs considerably decreased at 230 nm, indicating that PTX have been efficiently loaded into APS-PTX NPs. The FTIR outcomes (Fig. 2E) confirmed that the absorption peak of hydroxyl group stretching vibration of APS was at 3394.27 cm−1, and the vibrational absorption peak of free carboxyl group in APS galacturonic acid was at 1637.04 cm−1. The height at 1732.02 cm−1 corresponded to the carbonyl group (C = O) stretching vibration of PTX, whereas, within the APS-PTX NPs, the The height at 3394.27 cm−1 is shifted to 3376.35 cm−1, the absorption peak at 1637.04 cm−1 is shifted to 1645.04 cm−1 and the absorption peak at 1732.02 cm−1 is shifted to 1715.93 cm−1. These outcomes point out that the carbonyl band of PTX and the hydroxyl band of galacturonic acid and the methylated free carboxyl band in APS have undergone vital adjustments after meeting, suggesting that an meeting has occurred between APS and PTX. XRD evaluation was carried out to judge the crystalline construction of APS, PTX, and their bodily combination (Fig. 2H). PTX exhibited attribute diffraction peaks at 5.6°, 8.8°, 10.0°, 12.2°, 12.5°, 15.8°, 18.6°, and 19.6°, whereas APS confirmed no distinct peaks. The bodily combination retained the sharp peaks of PTX, whereas these peaks disappeared in APS-PTX NPs, demonstrating the amorphous state of PTX inside the NPs. This XRD sample shift additional confirmed the efficient encapsulation of PTX by APS, highlighting the protecting position of APS in sustaining PTX in an amorphous type.
Moreover, APS-PTX NPs demonstrated minimal drug leakage over a 60-day interval, as detailed in Desk S5 (Supporting Data). To evaluate their stability and biocompatibility inside the circulatory system, in vitro evaluations have been performed. Determine 2G illustrates that the particle measurement of APS-PTX NPs remained secure in PBS supplemented with 10% fetal bovine serum (FBS) at 37 °C for twenty-four h, suggesting their potential stability throughout blood circulation. Moreover, hemolysis assays have been carried out, with outcomes offered in Desk S6 and Determine S6. The hemolysis charge of APS-PTX NPs was constantly beneath 5%, and erythrocyte morphology remained unaffected, confirming negligible hemolytic toxicity on the examined concentrations. These findings underscore the favorable stability and security profile of APS-PTX NPs for systemic purposes. Lastly, the in vitro launch of APS-PTX NPs was carried out in PBS resolution containing 3% SDS (pH = 7.4). As proven in Determine S5, cumulative PTX launch from APS-PTX NPs was considerably decrease than that from PTX in any respect time factors, indicating a sustained launch conduct.
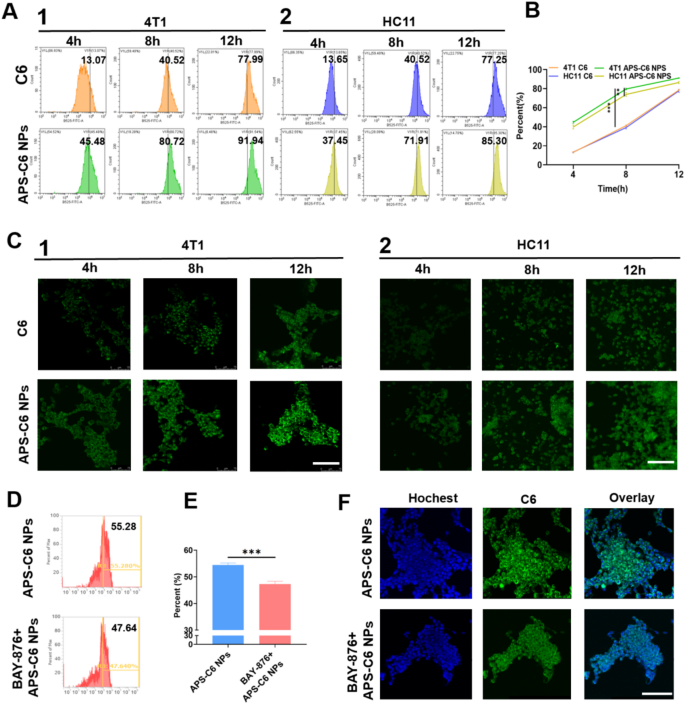
Cell uptake and focusing on impact of APS-PTX NPs. (A) Fluorescence depth outcomes of free C6 and APS-C6 NPs in 4T1(1) and HC11(2) cells was analyzed by FCM assay at 4, 8, 12 h after incubation, and the quantitative evaluation of FCM outcomes (B). (C) CLSM fluorescence photos outcomes of C6 in 4T1 and HC11 cells after free C6 and APS-C6 NPs therapy for 8 h. (D) Fluorescence depth and quantitative outcomes of 4T1 cells incubated with APS-C6 NPs and APS-C6 NPs after BAY-876 pretreatment at 8 h, and the quantitative evaluation of FCM outcomes (E). (F) CLSM fluorescence photos outcomes of C6 in 4T1 cells after APS-C6 NPs and APS-C6 NPs after BAY-876 pretreatment at 8 h. Scale bar 100 μm
In vitro cell uptake
To guage the focusing on functionality of APS-PTX NPs, coumarin 6 (C6)-loaded APS nanoparticles (APS-C6 NPs) have been efficiently synthesized. As depicted in Fig. 3A1, the APS-C6 NPs group displayed stronger and extra widespread inexperienced fluorescence alerts within the cytoplasm of 4T1 cells in comparison with the free C6 group, indicating enhanced mobile uptake effectivity. We additionally performed cell uptake assessments in HC11 cells (Mammary Epithelium) (Fig. 3A2), which confirmed no vital distinction in FITC fluorescence depth between HC11 and 4T1 cells totally free C6. Nonetheless, APS-PTX NPs exhibited considerably greater uptake in 4T1 cells than in HC11 cells (Fig. 3B), a discovering additional confirmed by FCM evaluation (Fig. 3C). As well as, tumor cells overexpress GLUT1 to Gas their fast proliferation and metabolism. APS is wealthy in glucose residues. We hypothesized that APS may selectively goal 4T1 cells by means of GLUT1. With a view to additional show that the tumor focusing on of APS-PTX NPs is correlated with GLUT, we used BAY-876, a extremely selective and cell-permeable GLUT1 inhibitor. At a secure focus, when BAY-876 blocked GLUT1 in 4T1 cells, the uptake charge of APS-PTX NPs dropped from 55.28 to 47.64% (Fig. 3D, E). The outcome was additional validated by FCM evaluation (Fig. 3F). This implies that APS-PTX NPs, having quite a few floor glucose residues, are endocytosed through GLUT1. APS can actively goal tumor cells by means of particular binding to GLUT1, reaching exact drug supply whereas lowering harm to regular tissues. Therefore, the findings collectively confirmed the efficacy of APS-PTX NPs in selectively goal tumor cells slightly than regular cells.
In vitro anti-tumor results of APS-PTX NPs
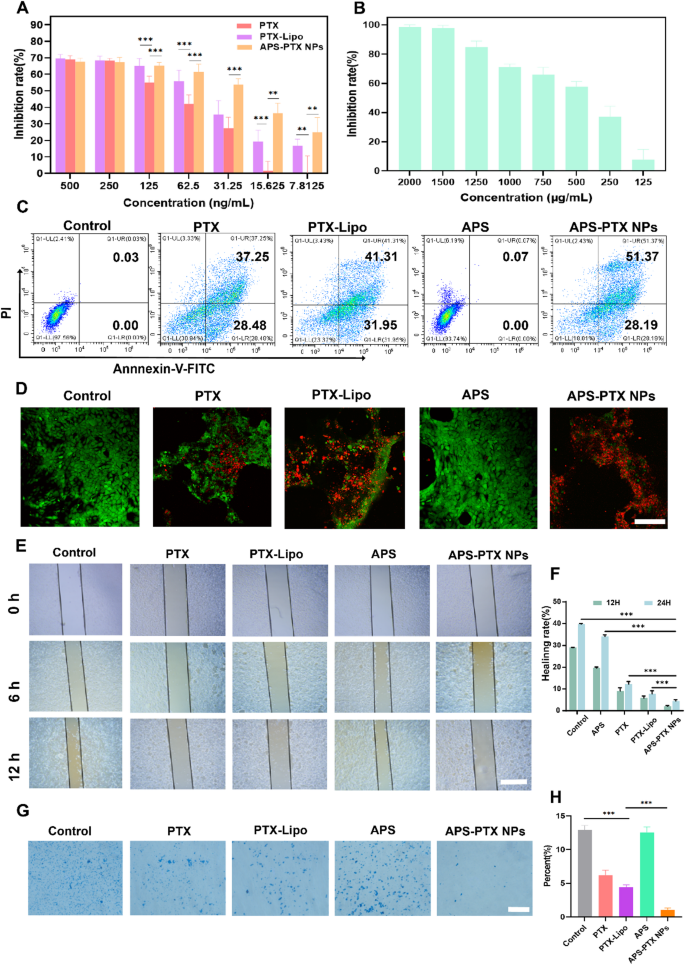
In vitro metastasis inhibition of APS-PTX NPs on 4T1 cell. (A) 4T1 cell viability. The cells have been incubated with varied concentrations of free PTX, PTX-Lipo and APS-PTX NPs for 48 h and the viability of cells was adopted detected by CCK8 (n = 3 samples per group). (B) 4T1 cell viability. The cells was incubated with varied concentrations of APS for 48 h and the viability of cells was adopted detected by CCK8. (C) Circulate cytometry expression of PI and Annexin V for apoptosis. (D) Stay/lifeless staining photos of 4T1 cells. (E) Consultant photos of scratch wound-healing assays on 4T1 cell after handled with free PTX, PTX-Lipo, APS and APS-PTX NPs. (F) Statistical outcomes of scratch wound-healing assays. (G) Consultant photos of transwell invasion assays on 4T1 cell after handled with free PTX, PTX-Lipo, APS and APS-PTX NPs. (H) Statistical outcomes of transwell invasion assays. Scale bar 100 μm (***p < 0.001, **p < 0.01, *p < 0.05)
The APS-PTX NPs promoted drug uptake by 4T1 cells, so we additional studied the impact of APS-PTX NPs on PTX cytotoxicity. To match the antitumor exercise of APS-PTX NPs with free PTX and the marketed preparation PTX-Lipo, we incubated 4T1 cells with APS, free PTX, PTX-Lipo, and APS-PTX NPs. The cytotoxicity of APS was weak, whereas the cytotoxicity of APS-PTX NPs was the strongest, considerably greater than that of free PTX and PTX-Lipo. The IC50 of free PTX, PTX-Lipo, and APS-PTX NPs have been 116.27 ± 8.08 ng/mL, 68.64 ± 2.15 ng/mL, and 42.14 ± 1.82 ng/mL, respectively (Fig. 4A and B). In response to the cell uptake outcomes, free PTX is taken up by cells by means of passive diffusion; PTX-Lipo enters cells by means of endocytosis and reveals stronger cytotoxicity, whereas APS-PTX NPs are taken up by cells by means of GLUT1-mediated endocytosis and thus exhibit the strongest cytotoxicity. As well as, we additionally analyzed the apoptosis of cells beneath totally different therapies. The outcome confirmed that APS didn’t take apparent impact, and the full apoptosis charges of APS, PTX, PTX-Lipo and APS-PTX NPs have been 0.10 ± 0.04, 63.51 ± 2.15%, 72.17 ± 1.20%, and 75.41 ± 3.75%, respectively (Fig. 4C and Determine S7). These outcomes have been in line with the developments noticed within the cytotoxicity experiments.
To evaluate cell viability and mitochondrial permeability, Calcein AM, a inexperienced fluorescent probe able to penetrating dwell cells, and propidium iodide (PI), a pink fluorescent dye particular to lifeless cells, have been utilized. Determine 4D illustrates the viability of 4T1 cells after 24 h of therapy with varied formulations. In comparison with the management group, the APS-treated teams exhibited minimal PI staining, indicating low cell demise. In distinction, the APS-PTX NPs group displayed the best variety of red-stained lifeless cells, aligning with the developments noticed in CCK-8 assays. These outcomes as soon as once more validate the glucose-targeting functionality supplied by APS.
Moreover, the impression of APS-PTX NPs on 4T1 cell migration and invasion was evaluated utilizing wound-healing and transwell assays. The migration potential of 4T1 cells was inhibited to various levels in wound-healing assay by totally different teams (Fig. 4E, F). In comparison with management group, the extent of wound therapeutic in different teams was lowered. Notably, the change of scratch gaps may barely be noticed in APS-PTX NPs group and relative charge of wound therapeutic have been 2.05 ± 0.61% and 4.46 ± 1.03% after 12 and 24 h respectively. And the speed of invasion had the same downward development as therapeutic charges after therapy (Fig. 4G, H). These outcomes indicated the mixture of APS-PTX NPs had a greater impact than free PTX and PTX-Lipo in inhibition of migration and invasion, and the benefit of APS as a nano-delivery provider in anti-tumor metastasis.
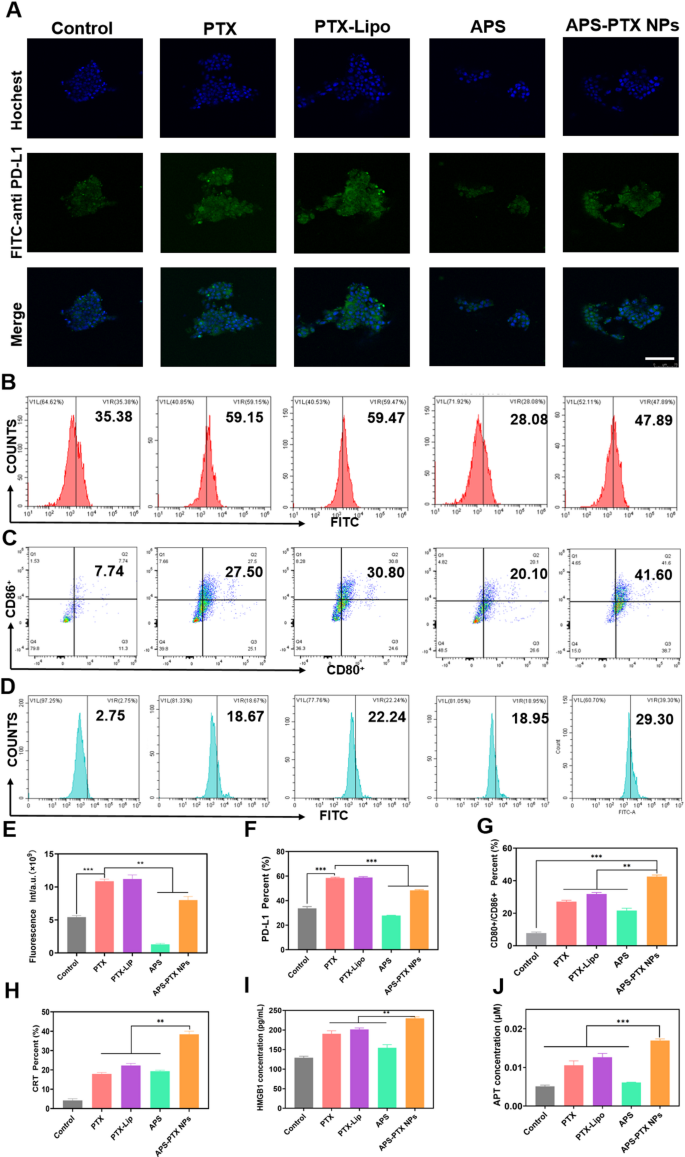
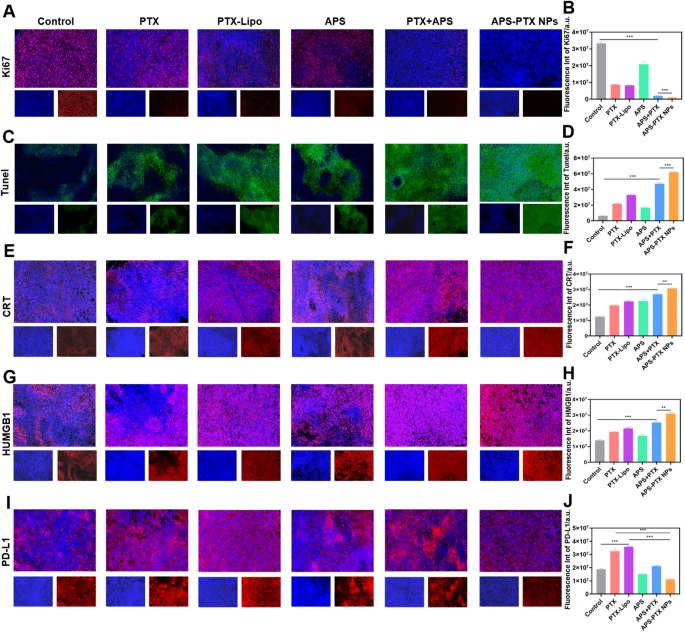
APS-PTX NPs cut back PD-L1 expression and induce ICD in vitro (A) CLSM fluorescence photos of PD-L1 within the tumor sphere after incubation by free PTX, PTX-Lipo, APS and APS-PTX NPs. The blue and inexperienced fluorescence represented hochest and anti-PD-L1 antibody, respectively. Scale bar: 100 μm. (B) Circulate cytometry photos of PD-L1 within the tumor sphere after incubation by free PTX, PTX-Lipo, APS and APS-PTX NPs. (C) Expression of CD80+/CD86+ on BMDCs after incubation with free PTX, PTX-Lipo, APS and APS-PTX NPs primarily based on circulation cytometry. (D) Expression of CRT on 4T1 after incubation with free PTX, PTX-Lipo, APS and APS-PTX NPs primarily based on circulation cytometry. (E) Semi-quantitative evaluation pink fluorescence of anti-PD-L1. (F) Quantitative evaluation of PD-L1 expression. (F) Quantitative evaluation of PD-L1 expression. (G) Quantitative evaluation of FCM outcomes of the expression of CD80+/CD86+ on DCs. (H) Quantitative evaluation of CRT expression. (I) HMGB1 secretion measured by HMGB1 assay kits (n = 3). (J) ATP secretion measured by ATP assay kits (n = 3). (***p < 0.001, **p < 0.01)
APS-PTX NPs induced ICD exercise and decreased PD-L1 in vitro
PTX, a extensively used chemotherapeutic agent, is understood to set off ICD in tumor cells [44]. To evaluate the flexibility of APS-PTX NPs to induce ICD, the externalization of CRT in 4T1 cells induced by APS-PTX NPs was first investigated utilizing circulation cytometry. Compared to the management group, PTX, PTX-Lipo, and APS-PTX NPs have been all able to considerably inducing CRT expression. Notably, the induction impact of APS-PTX NPs was markedly stronger than that of PTX-Lipo. This might doubtlessly be ascribed to the augmented endocytosis effectivity and elevated cytotoxicity, as depicted in Fig. 5D and H. Excessive mobility group field 1 protein (HMGB1) and adenosine triphosphate (ATP) have been quantified with the utilization of related kits. The discharge of HMGB1 and the secretion of ATP within the APS – PTX NPs group have been discovered to be greater than these within the different teams, as proven in Fig. 5I and J. These outcomes point out that APS-PTX NPs can induce ICD by means of the synergistic impact of APS and PTX and the improved endocytosis impact focusing on GLUT.
DCs are essential for triggering antigen – particular anti – tumor immune responses. They establish harm – related molecular patterns (DAMPs) which are discharged throughout ICD. Subsequently, DCs current the antigens to T cells, thereby enjoying a necessary half in kick – beginning the anti – tumor immune course of. Provided that APS-PTX NPs induce CRT publicity and the secretion of ATP and HMGB1 in tumor cells, their potential to stimulate DC maturation was Additional investigated. 4T1 cells have been handled with PBS, free PTX, PTX-Lipo, APS, and APS-PTX NPs, and their supernatants have been used to tradition bone marrow-derived dendritic cells (BMDCs). Circulate cytometry evaluation revealed that each one PTX-based therapies considerably enhanced DC maturation in comparison with the management, with APS-PTX NPs exhibiting probably the most potent impact (Fig. 5C, G). Notably, APS may additionally induce DC maturation, which can be associated to the discharge of DAMPs induced by APS. These outcomes are in line with the ICD detection.
Nonetheless, excessive expression of PD-L1 in breast most cancers tumor cells is commonly related to poor prognosis in sufferers [45, 46]. PTX can additional induce an upregulation of PD-L1 expression [47]. APS might ingeniously circumvent the disadvantage of PTX-induced PD-L1 upregulation. To exhibit whether or not APS-PTX NPs can cut back the PTX-induced upregulation of PD-L1 expression, 4T1 cells have been cultured with PBS, PTX, PTX-Lipo, APS, and APS-PTX NPs. Then, an anti-PD-L1 fluorescent antibody was added, and the samples have been detected by FCM. In contrast with the management group, PTX and PTX-Lipo may induce an upregulation of PD-L1 expression. APS may considerably cut back the PD-L1 expression on the floor of 4T1 cells. In contrast with free PTX and PTX-Lipo, APS-PTX NPs may considerably lower the PD-L1 expression (Fig. 5A, B, E, F). In response to earlier stories, APS inhibit PD-L1 expression in 4T1 cells through the AKT/mTOR signaling pathway [48]. Subsequently, we investigated the position of the AKT/mTOR/p70S6K signaling pathway within the discount of PD-L1 expression by APS and APS-PTX NPs by means of western blotting experiments. The outcomes confirmed that therapy with APS and APS-PTX NPs decreased the phosphorylation ranges of AKT, mTOR, and p70S6K, indicating that APS and APS-PTX NPs therapy downregulated the AKT/mTOR/p70S6K pathway in 4T1 cells (Determine S8). These findings counsel that the discount of PD-L1 by APS and APS-PTX NPs in tumor cells is related to the downregulation of the AKT/mTOR/p70S6K pathway. The above outcomes point out that APS, as a novel drug provider, can considerably cut back the PTX-induced excessive expression of PD-L1 on the floor of tumor cells, which isn’t achievable with frequent liposomes.
In vivo pharmacokinetics and tumor focusing on analysis of APS-PTX NPs
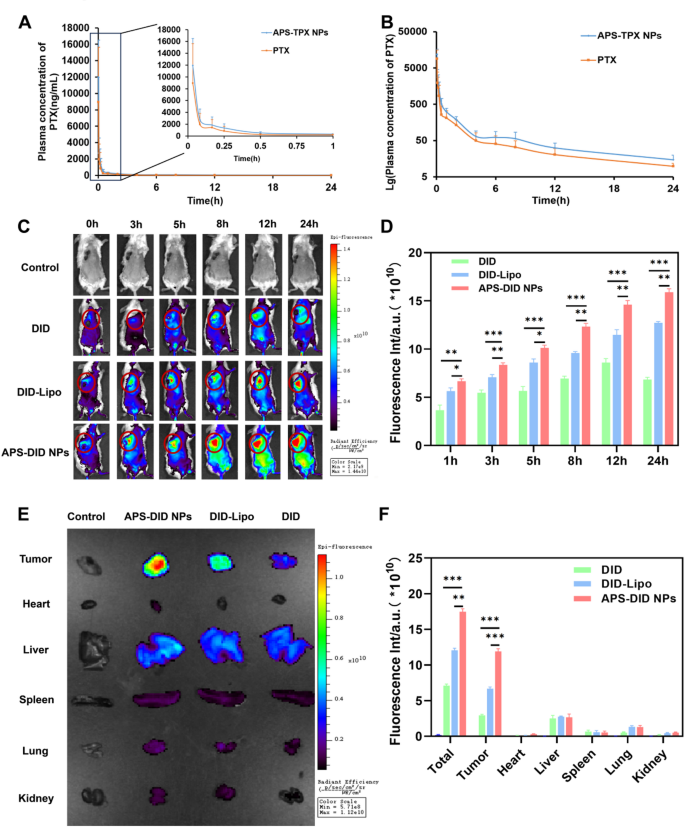
The drug focus–time curve of free PTX and APS-PTX NPs, and the focusing on potential in vivo and tissue biodistribution of APS-PTX NPs. (A) Blood concentration-time profile of PTX in rats after administration of free PTX and APS-PTX NPs (n = 6). (B) Lg blood concentration-time profile of PTX in rats after oral administration of free PTX and APS-PTX NPs (n = 6). (C) Fluorescence photos and quantitative evaluation (D) of 4T1 tumor-bearing mice after intravenous injection of DID, DID-Lipo and APS-DID NPs. (E) Fluorescence photos of tissues, together with tumor, coronary heart, Liver, spleen, lung, and kidneys after 4T1 tumor-bearing mice sacrificed at 24 h. (F) Fluorescence quantitative evaluation of tumors, coronary heart, liver, spleen, lung, kidney and all tissues. (***p < 0.001, **p < 0.01, *p < 0.05)
The fast clearance in systemic circulation and lack of tumor specificity usually necessitate excessive drug doses, resulting in opposed results. The in vivo focusing on potential and pharmacokinetic properties of APS-PTX NPs stay to be additional investigated. The affect of APS coating on the pharmacokinetics of PTX in nanoparticles was decided by monitoring the plasma drug focus within the physique and the outcomes have been proven in Fig. 6A, B and Tables S7 (Supporting Data). In contrast with free medication, pharmacokinetic parameters of PTX in APS-PTX NPs, comparable to common blood focus, peak focus Cmax, half-life t1/2, imply retention time (MRT 0−∞), and space beneath the curve (AUC0−∞) have been considerably improved or prolonged, highlighting the benefits of APS-PTX NPs. This additional signifies that APS as a nanocarrier can improve the in vivo lengthy circulation of PTX. The tumor focusing on potential and tissue biodistribution of APS-PTX NPs have been Additional evaluated in 4T1 tumor bearing mice. The fluorescence intensities of the tumor websites are proven in Figs. 6C and D. From 0 h to 24 h, the fluorescence intensities of every group repeatedly elevated. Particularly, solely a small quantity of free DID reached the tumor website. In distinction, the fluorescence depth of DID-Lipo on the tumor website was considerably greater than that of free DID, primarily attributed to the EPR impact. Furthermore, in contrast with DID-Lipo, ranging from 3 h, APS-DID NPs exhibited a stronger fluorescence sign on the tumor website because of the focusing on potential of APS. Moreover, at 24 h, the mice have been sacrificed, and related tissues and organs have been collected. The leads to Fig. 6E, F indicated that the fluorescence depth within the main organs (tumor, liver, coronary heart, spleen, lung, kidney and all organs) of every group was far decrease than that on the tumor website. The whole fluorescence depth of APS-DID NPs was the strongest, indicating that their clearance impact in vivo was the bottom. Amongst all the full fluorescence intensities, the ratio of APS-DID NPs to free DID was 2.45, whereas the ratio in tumor tissues was 4.03, which demonstrates that APS-DID NPs possess vital tumor-targeting potential. On the tumor website, the buildup of free DID was the bottom, adopted by DID-Lipo, and APS-DID NPs confirmed the best accumulation, with vital variations among the many teams. In contrast with the free DID group, the fluorescence depth of DID-Lipo was 2.26-fold greater, and that of APS-DID NPs was 4.03-fold greater. This implies that the GLUT-mediated focusing on can considerably improve the buildup of APS-DID NPs on the tumor website. In contrast with DID-Lipo, the fluorescence depth of APS-DID NPs was 1.78-fold greater, which was primarily because of the enhanced accumulation ensuing from the focusing on of APS to the tumor website. The above experimental outcomes exhibit that APS-DID NPs can actively goal the tumor website by means of APS, which is helpful for the therapy of nanoparticles on the tumor website.
In vivo anti-tumor and anti-metastasis efficacy of APS-PTX NPs
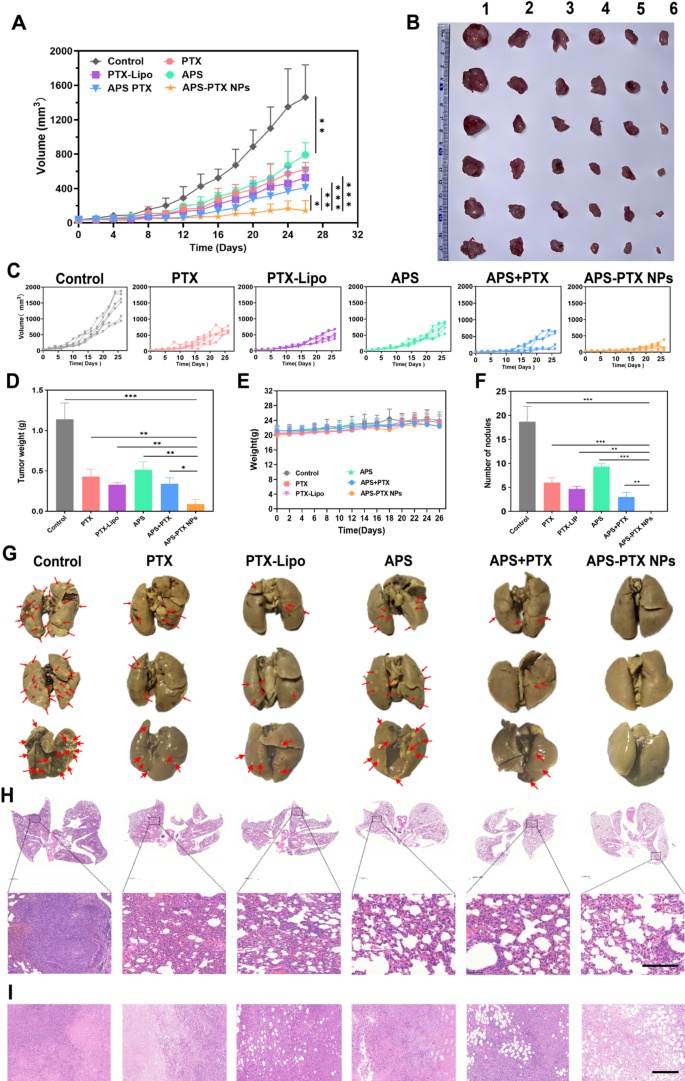
In vivo antitumor and anti-metastasis impact of systemic administration of APS-PTX NPs in 4T1 tumor-bearing mice (n = 6). (A) Tumor quantity progress curves resulting from in vivo antitumor results of various teams. (B) Pictures of the stripped tumors from the sacrificed mouse after the final administration (1: Management; 2: APS; 3: PTX; 4: PTX-Lipo; 5: APS + PTX; 6: APS + PTX NPs). (C) The particular progress curve of 4T1 tumor-bearing mice. (D) The tumor weights have been offered within the totally different therapy teams. (E) The physique weight of mice among the many totally different therapy teams. (F) Quantitative evaluation of the inhibition of node numbers. (G) Photographs of lungs in several teams and the pink arrows represented metastatic nodules on lungs. (H) H&E staining of lungs from 4T1 tumor-bearing mice within the 5 teams, and the darkish purple elements represented tumor metastasis. (I) H&E staining of tumor within the 5 teams (scale bar: 200 μm) (***p < 0.001, **p < 0.01, *p < 0.05)
In vivo antitumor efficacy was evaluated in tumor-bearing mice after therapy with PBS (management), PTX, PTX-Lipo, APS, APS + PTX and APS-PTX NPs. The expansion development of tumor quantity, tumor morphology and the tumor weight of every group have been in comparison with consider the antitumor efficacy (Fig. 7A–D). The tumor weight of the saline group was 1.14 ± 0.20 g. In comparison with PBS group, the tumor weight within the therapy teams all lowered. Particularly, the tumor weights of the PTX, PTX-Lipo, APS, APS + PTX and APS-PTX NPs teams have been 1.17 ± 0.46 g, 0.43 ± 0.09 g, 0.39 ± 0.08 g, 0.51 ± 0.10 g, 0.40 ± 0.12 g and 0.09 ± 0.06 g, respectively. The tumor quantity of the management, PTX, PTX-Lipo, APS, APS + PTX and APS-PTX NPs teams have been 1462.07 ± 376.59 mm3 619.08 ± 84.39 mm3 526.73 ± 122.35 mm3 719.88 ± 141.36 mm3 410.80 ± 232.21 mm3 and 143.03 ± 116.84 mm3 respectively, indicating a robust synergistic anti-tumor impact of the APS and PTX together. In contrast with the management group, APS confirmed a sure antitumor impact in vivo (54.90%), which was associated to the enhancement of immunity by APS [37]. More and more, each free PTX and PTX-Lipo teams exhibited stronger inhibition, the inhibition charges of free PTX and PTX-Lipo have been 62.39% and 71.16% relative to PBS, respectively. Particularly, APS-PTX NPs demonstrated probably the most potent inhibitory impact on tumor progress (92.28%), which was considerably greater than that of the bodily combination group of APS and PTX. This additional validates the benefits of APS-PTX NPs in exerting anti-tumor results through the EPR impact and GLUT-mediated focusing on. Furthermore, hematoxylin and eosin (H&E) (Fig. 7I), Ki67 (Fig. 8A, B), and terminal-deoxynucleotidyl transferase mediated nick finish labeling (TUNEL) (Fig. 8C, D) assay have been used to detect tumor tissue after therapy. In outcomes of the above assay, APS-PTX NPs additionally confirmed the very best effectivity accordingly, with critical apoptosis of the tumor cells, extra apparent destruction of tumor cell, and deformation or shrinking of nuclei, and a bigger share of the Ki67 positivity tumor sections when put next with different teams, which validated that APS-PTX NPs may inhibit tumor progress by inducing tumor necrosis and apoptosis, in addition to inhibiting proliferation.
Metastasis is the primary explanation for demise in TNBC sufferers, and 4T1 cells are extremely invasive and liable to lung metastasis. The metastasis inhibition of APS-PTX NPs was inspected, and the lung tissues have been rigorously collected and photographed on the finish of the experiment (Fig. 7G). The variety of seen metastatic nodules on the floor of lung tissue was recorded to judge its anti-metastasis impact (Fig. 7F). A lot of tumor metastases have been noticed on the floor of lung tissues within the management group (18.67 ± 3.21). The variety of metastatic lesions on the lung floor was considerably lowered in each the PTX group (6.00 ± 1.00) and the PTX-lipo group (4.67 ± 0.58). The floor construction of lung tissues within the APS-PTX NPs group was near regular, with virtually no metastases (0.00 ± 0.00). The outcomes of H&E staining of lung tissue sections additionally confirmed that APS-PTX NPs may considerably cut back the variety of metastases in lung tissues (Fig. 7H). Paraffin-embedded sections of the lungs from mice in every group have been subjected to immunohistochemical staining for MMP-9 and Vimentin. The outcomes demonstrated that the expression ranges of MMP-9 (Determine S9. A and C) and Vimentin (Determine S9. B and D) within the lung tissue areas of the APS-PTX NPs group have been the bottom. These findings point out that APS-PTX NPs can considerably cut back the invasiveness of tumor cells and suppress lung metastasis, which can be attributed to the synergistic impact and focusing on impact of APS and PTX, enabling the drug to penetrate into tumors and metastases.
In vivo immune response elicited by APS-PTX NPs
Immunotherapy effectivity of APS-PTX NPs was investigated after in vivo anti-tumor experiment. Moreover, the capability of APS-PTX NPs to induce ICD was assessed by measuring key immune-stimulatory markers, together with extracellular ATP secretion, cell floor publicity of CRT, and intracellular launch of HMGB1. These markers play important roles in recruiting immune cells and facilitating antigen presentation, thereby enhancing anti-tumor immune responses [49]. As proven by CLSM, the publicity degree of CRT in cells was demonstrated. As depicted in Fig. 8E, in line with the in vitro outcomes, all of the PTX therapy teams exhibited a rise in CRT publicity. APS alone may induce the publicity of CRT in tumor cells. Specifically, the publicity of CRT induced by APS-PTX NPs was considerably greater in comparison with that within the free PTX group and the PTX-Lipo group. Semi-quantitative evaluation Additional revealed that in contrast with the free PTX group and the PTX-Lipo group, the CRT publicity within the APS-PTX NPs group elevated by 55.58% and 37.30%, respectively (Fig. 8F). The discharge of HMGB1 was additionally proven by CLSM, as illustrated in Fig. 8G and H. APS alone successfully triggered the discharge of HMGB1, whereas APS-PTX NPs prompted probably the most vital launch of HMGB1. Semi-quantitative evaluation additional indicated that in contrast with the free PTX group and the PTX-Lipo group, the discharge of HMGB1 within the APS-PTX NPs group elevated by 62.13% and 46.48%, respectively. Subsequently, APS-PTX NPs possess the strongest potential to set off the discharge of immunostimulatory alerts. Subsequent, we investigated the expression of PD-L1 within the tumors of mice (Fig. 8I, J). In contrast with the management group, free PTX considerably upregulated the expression of PD-L1. Conversely, according to the outcomes of the in vitro examine, APS markedly lowered the expression of PD-L1. The relative degree of PD-L1 decreased by roughly 19.23% in contrast with that within the management group. In comparison with the free PTX group and the PTX-Lipo group, the relative ranges of PD-L1 within the tumor tissues of the APS-PTX NPs group decreased by roughly 65.88% and 69.08%, respectively. Subsequently, APS-PTX NPs induced efficient in vivo degradation of PD-L1 through the therapy.
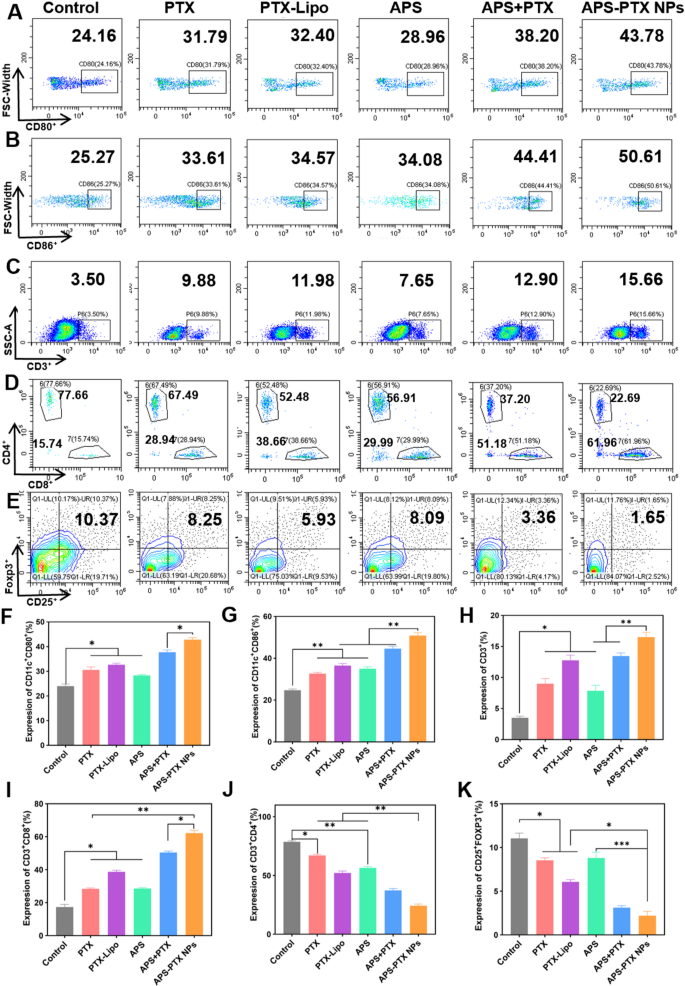
Immunological responses induced by totally different therapies in vivo. Circulate cytometric assay of the expression of A) CD80+ and B) CD86+ on DCs in tumor-draining lymph nodes. Circulate cytometric assay of (C) complete T lymphocytes (CD3+), D) cytotoxic T lymphocytes (CD3+CD8+) and helper T lymphocytes (CD3+CD4+), and E) Tregs (CD4+CD25+Foxp3+) in tumors. Quantitative evaluation of the expression of F) CD80+ and G) CD86+ on DCs, and H) complete T lymphocytes (CD3+), I) cytotoxic T lymphocytes (CD3+CD8+), J) helper T lymphocytes (CD3+CD4+), and Okay) Tregs (CD4+CD25+Foxp3+) in tumors. Information represents imply ± s.d (n = 3) (* p < 0.05, ** p < 0.01, *** p < 0.001)
DC maturation within the lymph nodes is a important course of to provoke immune response. As proven in Fig. 9A, B, F and G, all therapies containing free PTX, PTX-Lipo, APS, APS + PTX, and APS-PTX NPs, promoted DC maturation. The expression of CD80+ and CD86+ on the floor of DC within the lymphatic tissue in all teams turned greater. APS stimulated the maturation of DC as reported [50]. In our examine, APS considerably promoted the maturation of DC cells, with 28.86% (CD80+DC) and 34.08% (CD86+DC). The ultimate formulation successfully mixed APS and PTX, and delivered the drug to the tumor website with the next proportion to exert its efficacy. And the results of APS-PTX NPs and APS + PTX have been higher than these of PTX and PTX-Lipo. Amongst them, APS-PTX NPs group had highest proportion of mature DC cells, with 43.78% (CD80+DC) and 50.61% (CD86+DC), respectively. With the activation of mature DCs, the rise of helper CD4 optimistic T lymphocytes (CD4+T cell) and optimistic cytotoxic T lymphocyte (CD8+T cell) within the tumors have been noticed after therapy with APS-PTX NPs (Fig. 9D, I and J). Whereas the proportion of CD8+T cells in tumor tissue of every therapy group considerably elevated. In contrast with the management group, the proportion of CD8+T cells elevated by 2.9 occasions (APS-PTX NPs) or 0.9 occasions (APS).
On the opposite facet, relieving immunosuppression performs a decisive position in constructing the traditional antitumor immune barrier. Treg cells and M2 macrophages are key inhibitory cells within the immune system. The immune regulatory T cells (Treg cells) in tumor tissue have been Additional investigated to check the regulation of APS-PTX NPs to immunosuppressive cells. Relative to the management group, the ratio of Treg decreased to beneath 9% (APS) or 2% (APS-PTX NPs) (Fig. 8E and Okay).
Tumor related macrophages can also be an important class amongst immunosuppressive cells in TIME, primarily divided into intratumoral M2 macrophages (M2) with different activation, and immune-promoting M1 macrophages (M1) with classical activation recruit and activate adaptive immune cells. And the proportion of M1 elevated about 1.7 occasions (APS) and a couple of.6 occasions (APS-PTX NPs), and the proportion of M2 decreased, in contrast with management group (Determine S10 A-D). Mixture of APS and PTX utilizing certainly achieved decrease charge of M2/M1 than single use. These outcomes additionally confirmed that the proportion of immunosuppressive cells decreased considerably after APS-PTX NPs therapy.
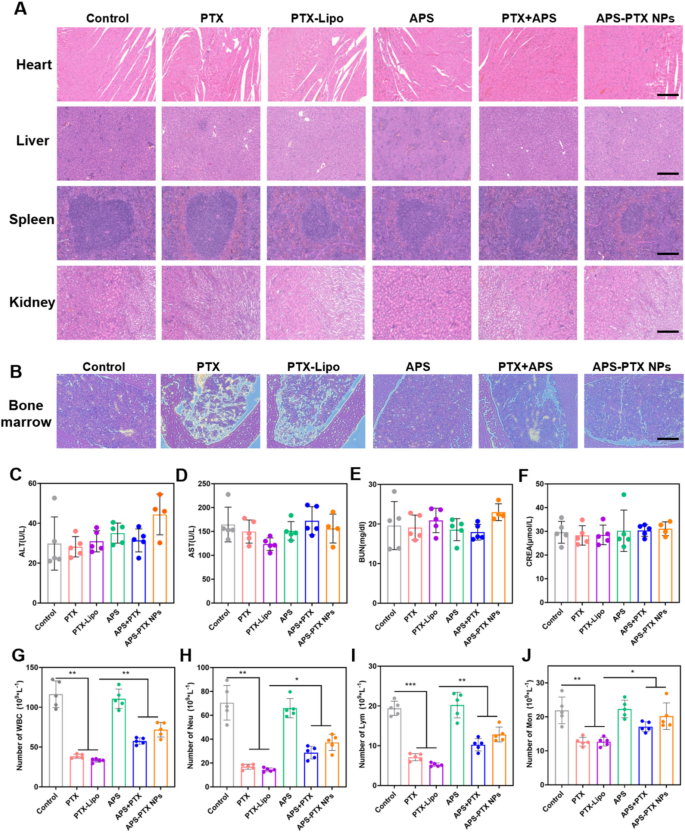
In vivo security of APS-PTX NPs
To guage the in vivo security of APS-PTX NPs, weight was monitored each different day and blood associated biochemical indexes have been measured on the finish of therapy (Fig. 10C-F). The load of mice in every group elevated usually with none vital fluctuations, indicating the protection of APS-PTX NPs (Fig. 7E). Furthermore, H&E staining photos of major organs from totally different therapy teams have been noticed with no pathological harm comparable to lesions or necrosis (Fig. 10A).
PTX, a member of the taxane household of chemotherapy medication, demonstrates excellent therapeutic efficacy towards a various vary of cancers, with a very pronounced impact on breast most cancers. It has been extensively adopted in medical oncological apply. However, the medical software of PTX is hampered by extreme opposed results, chief amongst which is myelosuppression. This limitation restricts its extra widespread utilization in most cancers therapy [30]. APS has been reported for use for bettering the myelosuppression of sufferers after chemotherapy [51,52,53]. Subsequently, we Additional decided the variety of white blood cells within the peripheral blood of mice in every group after administration. In contrast with the 4T1 management group, the variety of white blood cells within the blood of all mice handled with PTX was considerably lowered, nevertheless it was considerably improved after mixture therapy with APS (Fig. 10G). The analysis of differential white blood cell counts confirmed that in contrast with the management group, the numbers of neutrophils, lymphocytes, and monocytes within the free PTX group have been considerably decreased. In distinction, in contrast with the free PTX group, the numbers of neutrophils (Fig. 10H), lymphocytes (Fig. 10I), and monocytes (Fig. 10J) within the APS-PTX NPs group have been considerably elevated. To evaluate the impression of APS-PTX NPs on PTX-related bone marrow toxicity, histological alterations within the femur have been examined utilizing hematoxylin and eosin (H&E) staining. As depicted in Fig. 10B, therapy with free PTX and PTX-Lipo resulted in a discount in bone marrow cellularity and disrupted tissue structure. In distinction, APS administration successfully mitigated these opposed results, restoring bone marrow group and mobile density. These findings show the protecting position of APS towards PTX-induced bone marrow harm. These outcomes point out that in contrast with free PTX, APS-PTX NPs enhanced bone marrow hematopoiesis.
The in vivo security analysis of every group and the impact of therapies on the full and differential leukocyte counts. (A) The H&E staining of major organs (coronary heart, liver, spleen, kidney) from 4T1 tumor-bearing mice within the 5 teams (scale bar = 200 μm). (B) Histopathology adjustments of bone marrow detected by H&E staining. The evaluation results of ALT (C), AST (D), BUN (E) and CREA (F) in serum of 4T1 tumor-bearing mice after therapy. The evaluation results of WBC (G), Neu (H), Lym (I) and Mother (J) in peripheral blood of mice after therapy. (n = 5)