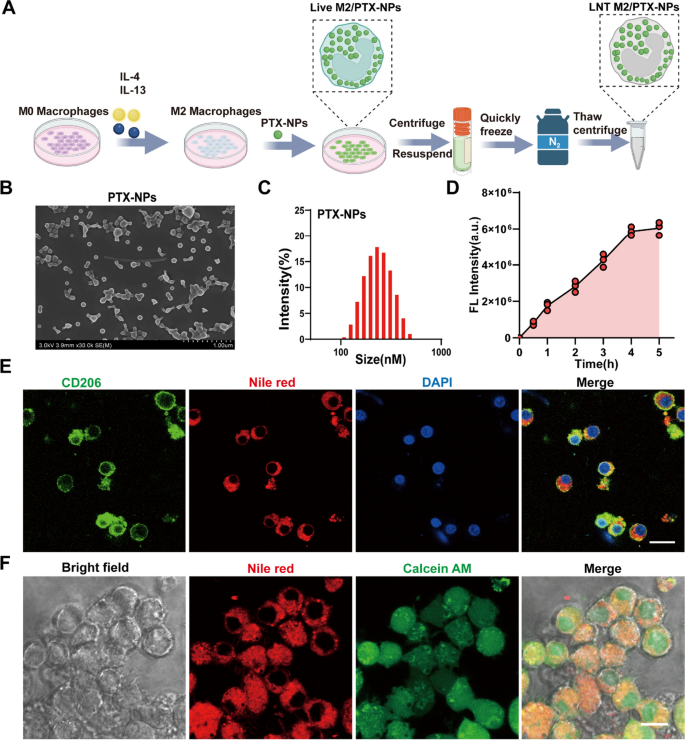
Preparation and characterization of PTX-NPs and reside M2/PTX-NPs
To enhance the solubility and bioavailability of PTX, we ready PTX-loaded PLGA nanoparticles (PTX-NPs) utilizing an emulsification technique. Scanning electron microscopy (SEM) confirmed that the PTX-NPs nanoparticles exhibited a homogeneous spherical morphology (Fig. 2B). Dynamic mild scattering (DLS) evaluation indicated that the PTX-NPs have a mean diameter of 241.32 ± 3.23 nm, a zeta potential measured at -19.63 ± 0.53, and a polydispersity index of 0.13 ± 0.006, indicating uniformity in measurement distribution (Fig. 2C and Supplemental Desk 1). Excessive-performance liquid chromatography (HPLC) decided that the encapsulation effectivity of PTX throughout the PLGA nanoparticles was 87.6 ± 3.06% (Determine S1 and Supplemental Desk 1).
Preparation and characterization of PTX-NPs and Reside M2/ PTX-NPs. A Schematic illustration of the preparation of LNT M2/PTX-NPs. B SEM pictures of PLGA nanoparticles loaded with PTX. C Hydrodynamic particle measurement distributions of PLGA nanoparticles loaded with PTX. D Quantitative evaluation of fluorescence depth reflecting the uptake of Nile crimson labeled PTX-NPs nanoparticles by M2 macrophages at completely different time factors (n = 3). E Immunofluorescence staining confirmed the profitable uptake of Nile crimson labeled PTX-NPs nanoparticles by the M2 macrophages. Scale bar 20 μm. F Cell viability of M2 macrophages, after co-incubation with Nile crimson labeled PTX-NPs nanoparticles, was assessed utilizing calcein-AM. Scale bar 10um
Given the effectiveness of vesicles and cell membranes derived from M2-type macrophages in mitigating irritation and modulating immunity inside SCI, we transformed NR8383 macrophages into the M2 phenotype to function carriers for loading our PTX-NPs nanoparticles. Circulate cytometry evaluation confirmed that after 48 h of publicity to IL-4 and IL-13, macrophages had been effectively polarized into the CD206⁺ M2 phenotype, attaining a polarization charge of 94.2% (Determine S2A). Moreover, qRT-PCR validated the profitable induction of M2 polarization by revealing a notable upregulation within the expression ranges of CD206, SOCS1, and Arg1 (Determine S2B–D). To trace the internalization of PTX-NPs by M2 macrophages, we labeled the nanoparticles with Nile Crimson. Confocal microscopy revealed that PTX-NPs uptake by M2 macrophages elevated progressively over 4 h after which plateaued (Determine S3 and Fig. 2D). Thus, a 4-h length was chosen as the following co-incubation time. Immunofluorescence staining consequence clearly demonstrated the profitable uptake of PTX-NPs by M2-polarized macrophages (Fig. 2E). Calcein AM staining of M2 macrophages that internalized PTX-NPs (known as Reside M2/PTX-NPs) confirmed that the nanoparticles had been primarily localized throughout the cytoplasm and didn’t have an effect on mobile viability (Fig. 2F).
Preparation and characterization of LNT M2/PTX-NPs
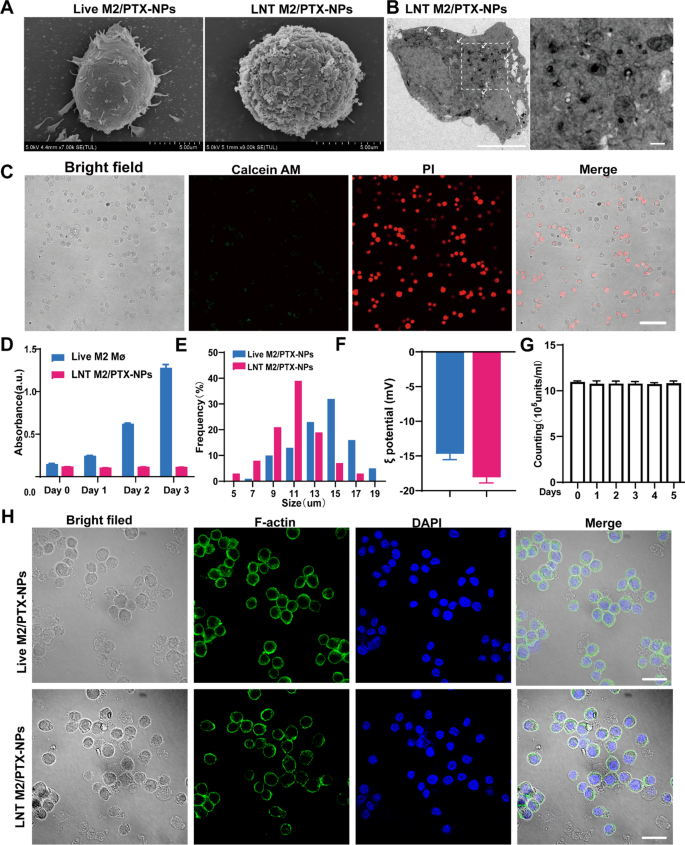
To forestall the potential reversion of nano-engineered reside M2 macrophages again to the M1 phenotype on the harm website, and to make sure the efficient PTX launch, M2 macrophages carrying PTX-NPs had been handled with liquid nitrogen for twenty-four h (Fig. 2A). Subsequently, they had been washed with PBS to acquire the LNT M2/PTX-NPs. SEM pictures revealed that liquid nitrogen remedy brought on the disappearance of filopodia on reside M2 macrophages, leading to a rougher cell floor (Fig. 3A). The transmission electron microscopy (TEM) pictures of LNT M2/PTX-NPs clearly present that PTX-NPs are internalized by M2 macrophages and situated within the cytoplasm (Fig. 3B). Notably, virtually all LNT M2/PTX-NPs cells had been positively stained with propidium iodide (PI) and never with calcein AM, indicating cell dying (Fig. 3C). Subsequent evaluation through a CCK-8 cell viability assay strengthened the discovering that LNT M2/PTX-NPs, post-liquid nitrogen processing, exhibited an absence of proliferative capability (Fig. 3D). Moreover, a minor discount within the diameter of LNT M2/PTX-NPs relative to their precursor was recognized (Fig. 3E). LNT M2/PTX-NPs exhibited a decrease zeta potential in comparison with their precursors, reside M2 macrophages enveloping PTX loaded nanoparticles (Reside M2/PTX-NPs), probably a results of membrane wrinkling and elevated permeability attributable to liquid nitrogen remedy (Fig. 3F). Via 5 days of morphological remark and particle measurement evaluation, we discovered that LNT M2/PTX-NPs displayed excessive stability, which additionally lays the muse for the sustained launch of PTX (Fig. 3G). Upon cytoskeletal staining with phalloidin, it was noticed that LNT M2/PTX-NPs cells preserved mobile buildings akin to these of their precursors-Reside M2/PTX-NPs (Fig. 3H). Utilizing HPLC evaluation, we discovered that the PTX content material in our cryo-shocked macrophage biomimetic drug supply system was roughly 12 μg per million models of each reside M2/PTX-NPs and LNT M2/PTX-NPs (Supplemental Desk 2). Additional, we aimed to discover whether or not liquid nitrogen remedy impacts the expression of proteins on the cell membrane. Sodium Dodecyl Sulphate–Polyacrylamide Gel Electrophoresis (SDS-PAGE) evaluation revealed that the protein profiles of LNT M2 (liquid nitrogen-treated M2 macrophages) and LNT M2/PTX-NPs carefully resembled these of reside M2 macrophages, indicating that almost all of proteins had been preserved (Determine S4). Subsequently, the biomedical security of LNT M2/PTX-NPs on microglial and neuronal cell traces was evaluated utilizing the CCK-8 assay. LNT M2/PTX-NPs exhibited no impact on the viability of PC12 and BV-2 cells following 24 h of incubation, even at elevated concentrations (1.0 × 10⁷ models mL⁻1), as proven in Determine S5.
Preparation and characterization of LNT M2/PTX-NPs. A Typical SEM pictures of reside M2//PTX-NPs cells and LNT M2/PTX-NPs. Scale bars 5um. B TEM pictures point out that PTX-NPs are internalized and situated within the cytoplasm of macrophages. Scale bars 5um. The size bar for enlarged pictures is 500 nm. C Viability evaluation of LNT M2/PTX-NPs performed utilizing the Calcein AM/PI staining. Calcein AM: reside cells; Propidium Iodide: useless cells. Scale bar represents 50 μm. D Cell viability evaluation of reside M2 and LNT M2/PTX-NPs by CCK-8 (n = 3). E Particle measurement distribution of LNT M2/PTX-NPs and its precursors. F The zeta-potential of LNT M2/PTX-NPs and its precursor (n = 3). G The soundness of LNT M2/PTX-NPs was evaluated by monitoring its quantitative modifications in PBS (37 °C) by cell counting (n = 3). H Structural composition of LNT M2/PTX-NPs and its precursor, with the cell nucleus highlighted utilizing DAPI (blue) staining, and the cytoplasmic F-actin marked by AF488 phalloidin (inexperienced). Scale bar 20um
In Vitro proinflammatory cytokine and chemokine elimination impact of LNT M2
Following SCI, an inflammatory cytokine storm and the intensive infiltration of immune cells precipitate a sustained inflammatory state and subsequent neurodegeneration [27,28,29]. Mitigating this irritation and lowering immune cell infiltration is essential for repairing the SCI-affected immune microenvironment.
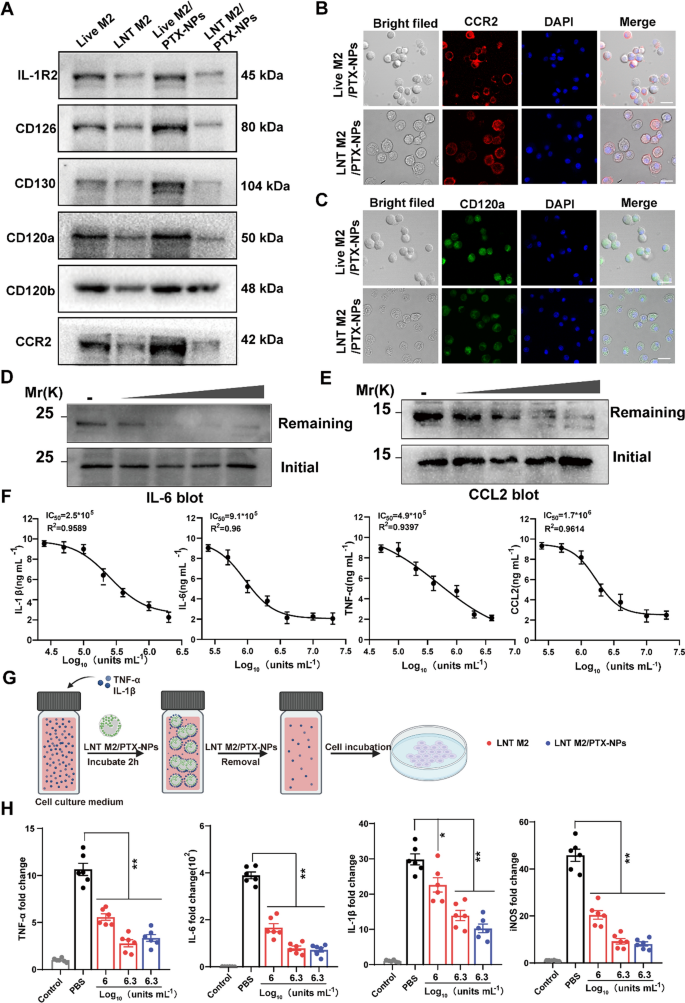
As beforehand proven, LNT M2/PTX-NPs retain a majority of proteins from their precursor cells, prompting us to additional examine the retention of cytokines receptors and chemokines receptors. Western blot (WB) outcomes confirmed that cytokine binding receptors (together with IL-R2 for binding IL-1β, CD126 and CD130 for binding IL-6, CD120a and CD120b for binding TNF-α) and the chemokine receptor CCR2(which binding CCL2) had been nicely preserved, with slight reductions in expression ranges (Fig. 4A). Confocal microscopy additionally confirmed clear labeling of CCR2 and CD120a on LNT M2/PTX-NPs, albeit with barely decrease fluorescence depth than their precursors (Fig. 4B–C).
The adsorption impact of LNT M2/PTX-NPs as a nano-decoy on inflammatory/chemotactic components. A Consultant protein expression of cytokine/chemokine receptors on LNT M2, LNT M2/PTX-NPs and their precursors had been detected utilizing western blotting. CCR2 (B) and CD120a (C) expression on Reside M2/PTX-NPs cells and LNT M2/PTX-NPs had been labeled with immunofluorescence staining and noticed by confocal microscopy. Scale bar 20um. Immunoblotting evaluation of the residual quantities of D IL-6 and E CCL2 after incubation with gradient concentrations of LNT M2/PTX-NPs (5 * 105 models mL−1, 1 * 106 models mL−1, 2 * 106 models mL−1, 4 * 106 models mL−1 respectively). F The adsorption impact of LNT M2/PTX-NPs to inflammatory components (IL-1β, IL-6, TNF-α) and chemokines (CCL2) was evaluated by means of ELISA. The y-axis indicating the concentrations of cytokines/chemokines left after publicity to LNT M2/PTX-NPs (n = 6). G An illustration depicting the method of cell stimulation utilizing a tradition medium infused with TNF-α and IL-1β. Previous to the incubation, the medium is handled with LNT M2 or LNT M2/PTX-NPs, that are subsequently eradicated by means of centrifugation. H The mRNA expression ranges of TNF-α, IL-1β, IL-6, and iNOS in main microglia cells had been measured utilizing qRT-PCR (n = 6). Error bars signify the SEM. p worth: *p < 0.05, **p < 0.01
To confirm the performance of those receptors, we launched rising quantities of LNT M2/PTX-NPs into PBS options containing TNF-α and CCL2, permitting interplay for two h. Western blot evaluation confirmed that, after elimination of LNT M2/PTX NPs, the residual ranges of TNF-α and CCL2 decreased with rising LNT M2/PTX-NPs, indicating dose-dependent adsorption of those inflammatory components (Fig. 4D, E).
We additional validated this adsorption impact utilizing ELISA, which demonstrated that LNT M2/PTX-NPs may dose-dependently adsorbed IL-1β, IL-6, TNF-α, and CCL2 (Fig. 4F). The IC50 values for the clearing results of LNT M2/PTX-NPs on IL-1β, IL-6, TNF-α, and CCL2 had been 2.5 × 105 models/mL, 9.1 × 105 models/mL, 4.9 × 105 models/mL, and 1.7 × 106 models/mL, respectively (Fig. 4F). Consequently, these outcomes verify that the receptors on LNT M2/PTX-NPs are purposeful, suggesting they’ll neutralize pro-inflammatory cytokines and inhibit extreme chemotaxis of peripheral immune cells post-injury.
Given the position of microglia activation and transformation into the pro-inflammatory M1 phenotype in mediating neural irritation after SCI [30], we evaluated whether or not LNT M2/PTX-NPs may inhibit this activation (Fig. 4G). IL-1β and TNF-α containing cell tradition medium had been pre-inculcated with LNT M2 or LNT M2/PTX-NPs for two h, then the supernatant was launched to microglial cultures. After 24 h, qRT-PCR confirmed that microglia within the PBS group considerably shifted in direction of the M1 phenotype with upregulated expression of TNF-α, IL-6, IL-1β, and iNOS (Fig. 4H). Notably, LNT M2 or LNT M2/PTX-NPs remedy considerably inhibited this phenotypic transformation, exhibiting a marked downregulation of those inflammatory markers (Fig. 4H). Collectively, this consequence means that LNT M2/PTX-NPs exhibit important neutralization effectivity throughout a number of cytokines and inhibits microglial M1 phenotype activation induced by cytokines.
LNT M2/PTX-NPs@Gel facilitates neural operate restoration in SCI rats
Gelatin Methacryloyl (GelMA) hydrogels, enriched with cell-adhesive RGD peptides, exhibit structural stability, biocompatibility, and injectability, making them superb for biomedical functions similar to tissue engineering and drug supply [31,32,33,34,35,36]. Particularly, GelMA demonstrates distinctive benefits within the remedy of SCI, the place it could fill the cavities shaped post-injury, guaranteeing direct supply of assorted bioactive substances to the lesion website [37, 38]. Its porous construction permits for the free motion of gear similar to oxygen, vitamins, and inflammatory/chemotactic components inside its matrix [39, 40].
On this research, we used GelMA because the structural platform to ship cryo-shocked M2 macrophages to spinal twine harm websites. We ready injectable LNT M2/PTX-NPs-loaded hydrogels (LNT M2/PTX-NPs@Gel) by mixing LNT M2/PTX-NPs with GelMA answer and irradiating it with 405 nm UV mild for 30 s. Moreover, PTX-NPs encapsulated in GelMA (PTX-NPs@Gel) and LNT M2 encapsulated in GelMA (LNT M2@Gel) had been ready utilizing the identical technique. The SEM pictures illustrated that PTX-NPs@Gel, LNT M2@Gel, and LNT M2/PTX-NPs@Gel all possessed a typical uniform and interconnected porous community, with the hydrogel pore sizes and buildings exhibiting no important variation upon the incorporation of PTX-NPs, LNT M2, or LNT M2/PTX-NPs (Determine S6A). Confocal microscopy confirmed uniform distribution of LNT M2/PTX-NPs throughout the hydrogel matrix (Determine S6B). In vitro drug launch research indicated a gradual, sustained launch of PTX for as much as 1 month (Determine S6C). Owing to the particularity of the SCI website, our supply system displays notable benefits attributable to its slow-release characteristic following a single administration. The biocompatibility of Gel, PTX-NPs@Gel, LNT M2@Gel, and LNT M2/PTX-NPs@Gel was additional confirmed by means of CCK-8 cell viability assays, reside/useless staining, and hemolysis exams, exhibiting minimal cytotoxicity (Determine S7).
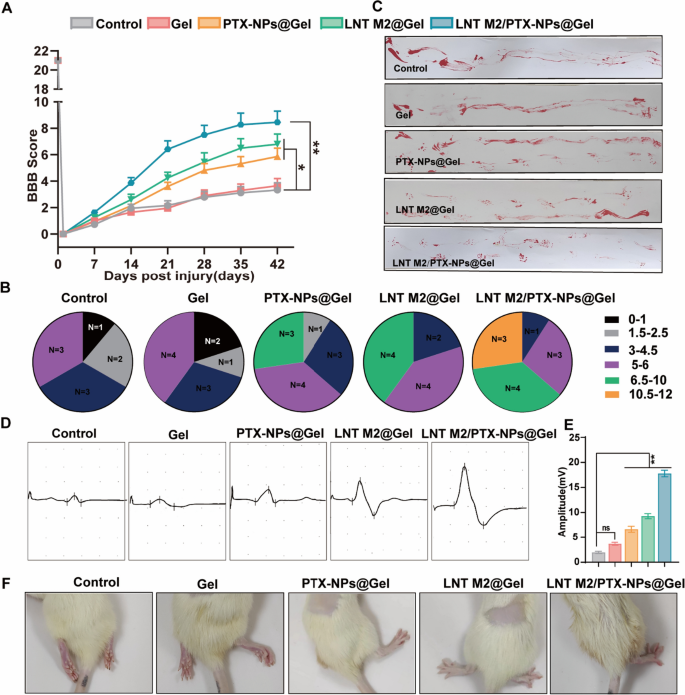
We additional evaluated the therapeutic efficacy of our system by injecting Gel, PTX-NPs@Gel, LNT M2@Gel, and LNT M2/PTX-NPs@Gel into the injured spinal cords of rats. BBB evaluation indicated important motor operate enchancment within the PTX-NPs@Gel and LNT M2@Gel teams 3 weeks post-treatment, whereas rats within the LNT M2/PTX-NPs @Gel group exhibiting notable enhancements 2 weeks post-treatment (Fig. 5A). It’s noteworthy that throughout the LNT M2/PTX-NPs group, 3 out of 11 rats introduced with steady plantar placement with assist and coordinated hindlimb actions, attaining BBB scores above 10, a situation not noticed in another group (Fig. 5B). Footprint evaluation revealed that rats within the LNT M2/PTX-NPs@Gel group exhibited probably the most important locomotor enchancment (Fig. 5C). PTX-NPs@Gel and LNT M2@Gel teams confirmed average enchancment, whereas Gel and Management teams exhibited continued hind limb dragging (Fig. 5C). This discovering was additional substantiated by photographic proof of the hind limbs in every group (Fig. 5F). Electrophysiological evaluation confirmed important enhancements within the Motor Evoked Potential (MEP) amplitudes within the PTX-NPs@Gel, LNT M2@Gel, and LNT M2/PTX-NPs@Gel teams relative to the Management group (Fig. 5D and E). The LNT M2/PTX-NPs@Gel group exhibited superior MEP amplitude enhancements relative to the opposite remedy teams (Fig. 5D and E). In abstract, the above findings counsel that LNT M2/PTX-NPs@Gel facilitates motor operate restoration after SCI and exerts important neuroprotective results.
The locomotor purposeful restoration of SCI rats underwent LNT M2/PTX-NPs@Gel remedy. A The BBB scoring in SCI rats over a interval of 6 weeks following varied remedies (n ≥ 9). B Distribution of BBB locomotion scores in several remedy teams at 6 weeks post-injury. C Footprint evaluation evaluating the restoration of hindlimb motor operate in SCI rats on the 6 weeks post-injury. D Electrophysiological evaluation of spinal twine injured rats utilizing MEP 6 weeks post-treatment. E Quantification of the motor evoked potentials (MEP) amplitudes (n = 6). F Consultant pictures of hind limbs of spinal twine injured rats 6 weeks after remedy. Error bars signify the SEM. p worth: “ns” means no important distinction, *p < 0.05, **p < 0.01
Transforming the spinal twine immune microenvironment with LNT M2/PTX-NPs@Gel in SCI rats
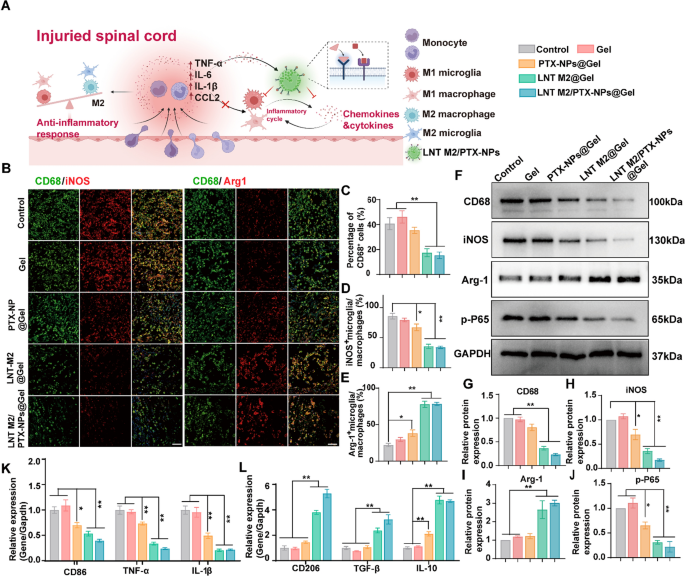
LNT M2/PTX-NPs demonstrated in vitro functionality as a nano-decoy for the elimination of inflammatory/chemotactic components, with this impact remaining comparable after incorporation into Gel (Determine S8). Subsequently, we additional investigated its inflammatory regulation efficacy in vivo utilizing an SCI rat mannequin. WB outcomes revealed a major suppressed expression of CD68 and the M1 macrophage/microglia marker iNOS within the LNT M2@Gel and LNT M2/PTX-NPs@Gel group, in comparison with the Management group (Fig. 6F–H). Moreover, a major improve within the expression of the M2 microglia marker Arg-1 was noticed in LNT M2@Gel and LNT M2/PTX-NPs@Gel group (Fig. 6F and I). The expression of phosphorylated p65 (p-P65), which promotes the manufacturing of irritation, was additionally considerably inhibited within the LNT M2@Gel and LNT M2/PTX-NPs@Gel remedy teams (Fig. 6F and J).
LNT M2/PTX-NPs@Gel attenuate neuroinflammation by inhibiting the infiltration of immune cells and shift M1/M2 polarization in SCI rats. A Schematic diagram illustrates the mechanism of LNT M2/PTX-NPs in inhibiting infiltration of immune cells and modulating M1/M2 polarization. B Immunofluorescence co-staining of CD68 (inexperienced) with iNOS (crimson) and Arg1 (crimson) in SCI rats with completely different remedies at 7 days post-injury. Scale bar 50 μm. C Quantitative evaluation of CD68+ microglia/macrophage infiltration within the harm website of SCI rats with completely different remedies (n = 4). Quantitative evaluation of immunofluorescence staining for the proportion of iNOS + microglia/macrophages (D) and Arg-1 + microglia/macrophages (E) in Determine B (n = 4). F Western blotting evaluation of CD68, iNOS, Arg-1, and p-P65 expression in spinal twine lesion areas at 7 days after harm. G–J Quantitative evaluation of CD68, iNOS, Arg-1, and p-P65 protein expression in F (n = 3). mRNA expression ranges of (Okay) M1 sort markers (CD86, TNF-α, IL-1β) and L M2 sort markers (CD206, TGF-β, IL-10) in SCI rats with completely different remedies had been analyzed by qRT-PCR (n = 6). Error bars signify the SEM. p worth: *p < 0.05, **p < 0.01
To evaluate the infiltration of immune cells and the polarization states of microglia/macrophages in remedy teams, we additional applied immunofluorescence co-staining of CD68 with iNOS and Arg1, respectively. The outcomes confirmed that LNT M2@Gel and LNT M2/PTX-NPs@Gel administration considerably diminished CD68-positive cells, indicating suppressed immune cell infiltration (Fig. 6B, C). This suppression probably outcomes from the adsorption of chemotactic components by receptor on the cryo-shocked M2 macrophages (Fig. 6A). There was a marked discount within the proportion of iNOS+ cells in LNT M2@Gel and LNT M2/PTX-NPs@Gel teams steered a diminished proportion of M1 phenotype microglia/macrophages on the lesion website (Fig. 6B and D). qRT-PCR analyses confirmed this by exhibiting important downregulation within the expression of M1-associated genes (CD86, TNFα, IL-1β) (Fig. 6Okay). Conversely, there was a rise in Arg1+ cells, indicating an elevated proportion of M2 phenotype microglia/macrophages within the LNT M2@Gel and LNT M2/PTX-NPs@Gel teams (Fig. 6B and E). This was additional substantiated by the upregulation of M2-specific genes (CD206, TGF-β, IL-10) (Fig. 6L).
In conclusion, our outcomes point out that LNT M2/PTX-NPs@Gel inhibits inflammatory infiltration and modulates the stability between anti-inflammatory and pro-inflammatory phenotypes post-SCI by means of the adsorption motion of receptors retained on the membrane of cryo-shocked M2 macrophages.
LNT M2/PTX-NPs@Gel enhances axonal regeneration in rats with SCI
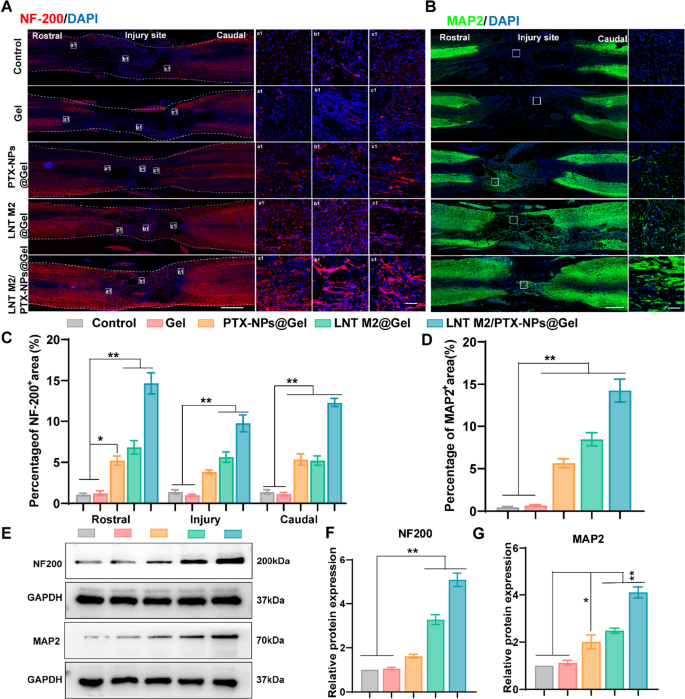
Practical restoration after SCI is basically decided by the regeneration of broken axons and the formation of latest synaptic connections [41, 42]. Subsequently, immunofluorescence staining for neurofilament protein (NF-200) and microtubule-associated protein 2 (MAP2) was employed to look at nerve progress and axon regeneration in several remedy teams. The Management and Gel-only teams displayed negligible NF-200 and MAP2 staining on the heart of harm (Fig. 7A–D). PTX-NPs@Gel and LNT M2@Gel implanted teams confirmed a marked improve in NF-200 and MAP2 staining throughout the injured areas, suggesting enhanced axonal regeneration (Fig. 7A–D). Earlier research have indicated that inflammatory cytokines promote the activation of astrocytes, which in flip facilitates the formation of glial scars, thereby impeding neural regeneration within the injured space [43,44,45]. We speculate that the axonal regenerative impact of LNT M2 is attributed to its important anti-inflammatory properties. The mix of PTX and LNT M2 demonstrated a synergistic impact, with a major infiltration of NF200+ axons into the lesion space within the LNT M2/PTX-NPs@Gel group, and regenerative axonal fibers filling your entire lesion space, virtually utterly bridging the 2 broken ends (Fig. 7A and C). Equally, the density of MAP2-positive neurons throughout the lesion space of the LNT M2/PTX-NPs@Gel group considerably exceeded that of different teams (Fig. 7B and D). Western blot evaluation for NF200 and MAP2 expression additional corroborated these observations (Fig. 7E–G). Total, the findings display that implantation of our cryo-shocked M2 macrophage based mostly multifunctional scaffold considerably promotes neural regeneration in SCI rats.
LNT M2/PTX-NPs@Gel promotes axonal regeneration within the injured spinal twine. A Consultant immunostaining pictures of NF200 (crimson) for SCI and every remedy group at 6 weeks post-injury, together with native pictures of the rostral border (a1), epicentral (b1), and caudal border (c1) boundaries of the injured spinal twine. Scale bar = 1000um. The size bar for enlarged pictures is 50 μm. B Consultant immunostaining pictures of MAP2 (inexperienced) for SCI and every remedy group at 6 weeks after harm. Scale bar = 1000um. The size bar for enlarged pictures is 50 μm. C Quantification of NF200 (crimson) optimistic alerts in rostral, epicentral, and caudal of the lesion space (n = 4). D Quantification of MAP2 optimistic alerts (inexperienced) within the lesion space (n = 4). E–G Western blotting and quantitative evaluation of NF200 and MAP2 expression ranges in spinal twine lesion space in several teams (n = 3). Error bars signify the SEM. p worth: *p < 0.05, **p < 0.01
LNT M2/PTX-NPs@Gel implantation reduces scar formation in rats with SCI
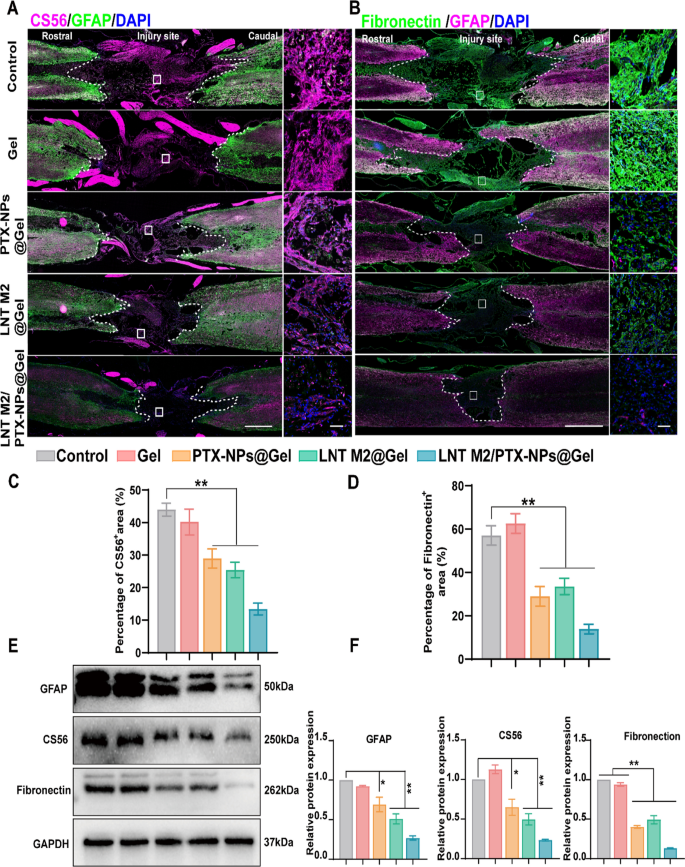
Following SCI, activated astrocytes progressively accumulate across the harm core, forming glial scars [46]. These glial scars act as a pure barrier and concurrently secrete inhibitors of neural regeneration similar to CSPG, thus hindering axonal regeneration post-SCI [47]. Moreover, fibroblasts proliferate, migrate, and collect on the harm core to kind fibrotic scars represent one other important reason for axonal regeneration failure and purposeful deficits [48, 49]. We used GFAP (astrocyte marker) to characterize glial scar formation and CS56 to indicate CSPG within the lesion space. Fibronectin, a key part of fibrotic scars, was additionally characterised by immunofluorescence staining. Intensive CS56 and Fibronectin alerts had been noticed within the harm areas of the Management and Gel-treated teams, together with widespread GFAP-positive reactive astrocytes across the harm edge (Fig. 8A–D and Determine S9).
LNT M2/PTX-NPs@Gel inhibited glial and fibrous scar formation following SCI. A GFAP/CS56 immunofluorescence co-staining was employed to depict the deposition of glial scar elements on the harm website. Scale bar = 1000um. The size bar for enlarged pictures is 50 μm. B GFAP/Fibronectin immunofluorescence co-staining was employed to depict the fibronectin-positive fibrotic scar elements on the harm website. Scale bar = 1000um. The size bar for enlarged pictures is 50um. Quantitative evaluation of CS-56 (C) and Fibronectin (D) optimistic alerts in every group (n = 3). E, F Western blotting and quantitative evaluation of GFAP, CS56 and Fibronectin expression ranges in spinal twine lesion space in several teams (n = 3). Error bars signify the SEM. p worth: *p < 0.05, **p < 0.01
PTX administration was noticed to inhibit glial scar formation and cut back the deposition of CSPG, which is basically in accordance with earlier stories (Fig. 8A, C and Determine S9) [23]. Equally, LNT M2 considerably diminished glial scar formation and CSPG deposition, as evidenced by a lot weaker alerts of each GFAP and CS56 in LNT M2@Gel group (Fig. 8A, C and Determine S9). As well as, implantation of PTX or LNT M2 on the lesion website additionally considerably diminished fibrotic scar density (Fig. 8B and D). Moreover, the mixed use of PTX and LNT M2 confirmed a synergistic impact, resulting in the bottom expression of CSPG, fibronectin, and GFAP within the LNT M2/PTX-NPs@Gel group, indicating an enhanced suppression of each glial and fibrotic scar formation (Fig. 8A–D and Determine S9). Outcomes from western blot analyses had been in keeping with the information obtained by IF, additional confirming our findings (Fig. 8E, F). In conclusion, the cryo-shocked M2 macrophages-loaded scaffold we developed, leveraging the mixed results of PTX and LNT M2, considerably suppresses glial scar hyperplasia and prevents fibrotic scars deposition within the lesion space.
In vivo biocompatibility evaluation
To evaluate in vivo biocompatibility, histological analyses of main organs and blood exams had been carried out on the 6 weeks post-injury. As anticipated, histological evaluation revealed no important injury to the foremost organs, and there have been no will increase in alanine aminotransferase (ALT), aspartate transaminase (AST), blood urea nitrogen (BUN), or creatinine (CRE) ranges in any of the teams. (Determine S10). Collectively, these outcomes counsel passable biocompatibility of the implant hydrogels in vivo.
Complete analysis of LNT M2/PTX-NPs@Gel in SCI remedy utilizing bioinformatics
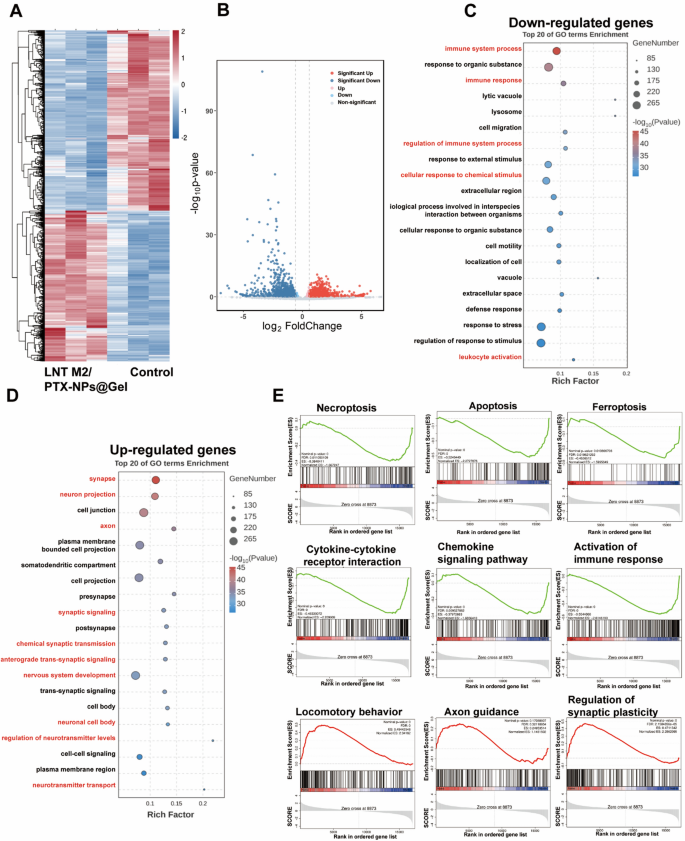
To additional examine the mechanisms by which our cryo-shocked macrophage derived scaffold aids within the neural purposeful restoration of SCI rats, transcriptomic analyses had been performed. A complete of 1501 differentially expressed genes had been recognized (absolute Fold change ≥ 1.5, p < 0.05), with 682 genes upregulated and 819 downregulated following remedy with LNT M2/PTX-NPs@Gel (Fig. 9A, B). Gene Ontology (GO) evaluation revealed that remedy with LNT M2/PTX-NPs@Gel decreased inflammation-related signaling pathways. Particularly, in contrast with the Management group, the down-regulated genes within the LNT M2/PTX-NPs@Gel remedy group had been enriched in immune system course of, immune response, regulation of immune system course of, mobile response to chemical stimulus, and leukocyte activation (Fig. 9C). This remark is extremely in keeping with our in vitro and in vivo outcomes, demonstrating that LNT M2/PTX-NPs@Gel mitigates neuroinflammatory responses and curtails the infiltration of inflammatory cells. Notably, genes upregulated in LNT M2/PTX-NPs@Gel group had been enriched in pathways associated to neurofunctional regulation, similar to synapse, neuron projection, axon, synaptic signaling, chemical synaptic transmission, anterograde trans-synaptic signaling, nervous system growth, neuronal cell physique, regulation of neurotransmitter ranges, and neurotransmitter transport (Fig. 9D). These findings additionally validate our earlier outcomes relating to the neuroprotective properties and axonal regeneration promotion by LNT M2/PTX-NPs@Gel.
Main transcriptional modifications in SCI rats with LNT M2/PTX-NPs@Gel remedy. A Heatmap and volcano plot (B) exhibiting differentially expressed genes within the spinal twine lesion space between the Management group and the LNT M2/PTX-NPs@Gel remedy group (Fold change > 1.5 and p worth < 0.05). GO evaluation of downregulated genes (C) and up-regulated genes (D) within the spinal twine lesion space from Management and LNT M2/PTX-NPs@Gel remedy group. E Gene set enrichment evaluation (GSEA) exhibiting enrichment of genes units concerned in necroptosis, apoptosis, ferroptosis, cytokine-cytokine receptor interplay, chemokine signaling pathway, activation of immune response, locomotory habits, axon steerage, regulation of synaptic plasticity in LNT M2/PTX-NPs@Gel group in comparison with Management group
Gene set enrichment evaluation (GSEA) revealed that, in comparison with the Management group, the LNT M2/PTX-NPs@Gel group confirmed downregulation of genes concerned in necrosis (ES = − 0.38), apoptosis (ES = − 0.52), and ferroptosis (ES = − 0.45), indicating that LNT M2/PTX-NPs@Gel has a protecting impact in opposition to neural dying (Fig. 9E). Moreover, a collection of genes associated to cytokine-cytokine receptor interplay (ES = − 0.48), chemokine signaling pathway (ES = − 0.38), and activation of immune response (ES = − 0.55) had been downregulated within the LNT M2/PTX-NPs@Gel group in contrast with the Management group, confirming its results in inhibiting neuroinflammation (Fig. 9E). LNT M2/PTX-NPs@Gel group exhibited upregulated expression of genes concerned in locomotory habits (ES = 0.49), axon steerage (ES = 0.25), and regulation of synaptic plasticity (ES = 0.47), highlighting its potential in supporting motor operate restoration (Fig. 9E). These mRNA sequencing outcomes assist our experimental findings, demonstrating that LNT M2/PTX-NPs@Gel displays anti-neuroinflammatory results and exerts neuroprotective actions in rats with SCI.