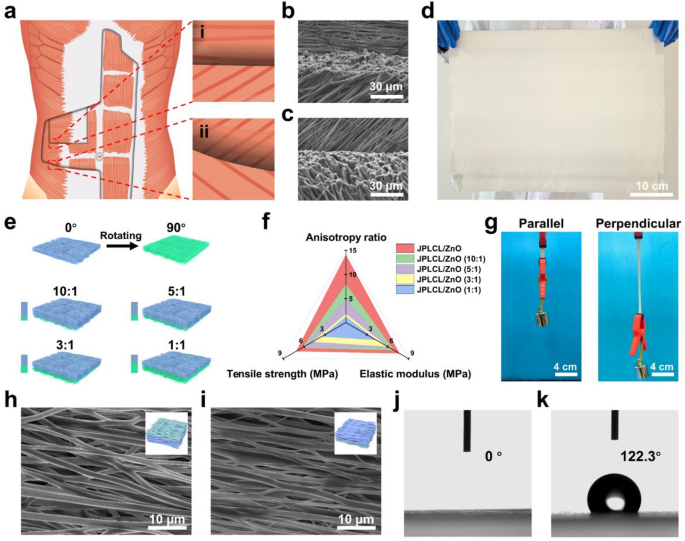
PLCL was chosen for electrospinning as a result of its good flexibility, biodegradability, and biocompatibility [35, 36]. PLCL/ZnO patch was fabricated by way of multi-channel electrospinning of a polymer combination containing PLCL, ZnO nanoparticles, and I-2959 photoinitiator. Subsequently, PLCL/ZnO patch was immersed in MPC answer to develop a PMPC coating on every fiber of the highest floor by UV curing, yielding JPLCL/ZnO patch. Bioinspired by the anisotropic structure of human belly wall, JPLCL/ZnO patch with a biomimetic muscle fiber-like morphology was fabricated by adjusting fiber orientation throughout electrospinning (Fig. 2a). As proven in Fig. 2b and c, our patch displays a morphology with fiber alignment much like that of muscle fibers, with fibers aligned at 0°, 45°, and 135°, similar to the transversus abdominis, inner indirect, and exterior indirect muscular tissues, respectively. Furthermore, our patch might be produced at a big scale (Fig. 2d), demonstrating its potential for medical translation. These outcomes display that our patch with a biomimetic muscle fiber-like morphology has been efficiently fabricated.
In medical apply, designing patches that match the mechanical anisotropy of pure belly wall is essential for defect restore, as mechanical mismatch may cause poor tissue integration, postoperative adhesion, and hernia recurrence [31, 34, 37]. Usually, patches used for the restore of belly wall defect in numerous areas are anticipated to own region-specific anisotropy ratios (the mechanical property ratio within the coronal axis and vertical axis). To judge the mechanical anisotropy of our patches, stress-strain curves of JPLCL and JPLCL/ZnO patches had been analyzed beneath tensile loading utilized both parallel or perpendicular to the fiber orientation (Determine S1). Each patches exhibit vital anisotropy, with the parallel route exhibiting considerably larger tensile energy and elastic modulus than the perpendicular route (Determine S2). Particularly, in contrast with JPLCL patch, JPLCL/ZnO patch demonstrates a 61% improve in tensile energy (7.71 vs. 4.79 MPa) and 174% improve in elastic modulus (7.14 vs. 2.60 MPa) within the parallel route (Determine S2). The improved mechanical properties are attributed to the incorporation of ZnO nanoparticles, which act as inflexible nanofillers within the PLCL polymer matrix to enhance load switch, limit polymer chain mobility, and thereby improve tensile energy [38,39,40]. To satisfy the mechanical anisotropy necessities of numerous areas of the belly wall, the mechanical properties of our JPLCL/ZnO patches might be modified in keeping with the precise utility necessities by adjusting the orientation stacking of the fibers. By adjusting the quantity ratios of the polymer combination electrospun within the 0° and 90° instructions from 1:1 to 10:1 (Fig. 2e and Desk S1), JPLCL/ZnO patches with completely different orientation stacking ratios (i.e., 10:1, 5:1, 3:1, and 1:1) had been fabricated and their mechanical properties had been investigated. Our JPLCL/ZnO patches can obtain anisotropy ratios starting from roughly 1 to 14 (Fig. 2f and Determine S3), which might meet the total spectrum of belly wall anisotropy necessities (a spread of 1–9) [29, 30]. Furthermore, the tensile energy (4.39–7.71 MPa) and elastic modulus (3.49–7.14 MPa) of those patches meet or exceed the baseline necessities (0.08 MPa for tensile energy [31, 32] and 0.04 MPa for elastic modulus [41]) for belly wall defect restore (Fig. 2f). Along with fiber orientation, ZnO content material influences the mechanical efficiency of JPLCL/ZnO patches. As proven in Determine S4, JPLCL/ZnO patches with larger ZnO contents exhibit elevated tensile energy and elastic modulus, indicating the reinforcing function of ZnO nanoparticles within the PLCL matrix. Contemplating the anisotropic gradient distribution traits of human belly wall, now we have chosen JPLCL/ZnO patch with 5 wt% ZnO and the very best anisotropy because the consultant pattern for our examine to analyze the results of structural anisotropy on mechanical efficiency and tissue regeneration. Below a 200 g load, JPLCL/ZnO patch displays higher elongation within the perpendicular route than within the parallel route (Fig. 2g), demonstrating its excessive flexibility and mechanical anisotropy. Due to this fact, by adjusting the orientation stacking of the fibers, JPLCL/ZnO patches possess tunable anisotropy ratios, tensile energy, and elastic modulus, permitting them to adaptively match the mechanical necessities of pure belly wall.
The fiber alignment of porcine belly wall muscle was evaluated by optical microscope. As proven in Determine S5, the porcine belly wall displays an aligned fibrous structure with seen fiber bundles. To additional assess fiber morphology, tissue samples had been harvested and sectioned in two instructions for HE staining: longitudinal part (parallel to the muscle fiber route) and cross part (perpendicular to the fiber route). As proven in Determine S6, the muscle fibers are extremely aligned with a mean diameter of roughly 50 μm, which is in line with reported diameters of porcine muscle fibers with a spread of 47–98 μm [42, 43]. Impressed by the anisotropic structure of the belly wall, now we have fabricated JPLCL and JPLCL/ZnO patches to imitate its native construction for defect restore. The morphologies of those patches had been characterised by scanning electron microscopy (SEM). JPLCL and JPLCL/ZnO patches exhibit fibrous morphologies, with an elevated fiber diameter on the highest floor (PMPC-coated floor) in comparison with the underside floor following the deposition of the PMPC coating (Fig. 2h and that i). Orientation evaluation additional reveals that almost all fibers are aligned inside a slim deviation of −20° to twenty° (Figures S7 and S8), indicating the extremely oriented fibrous construction. The fiber orientation noticed in our JPLCL/ZnO patch carefully mimics the extremely aligned construction of porcine belly wall muscle, which is essential for mimicking the anisotropic traits of pure belly wall within the utility of defect restore.
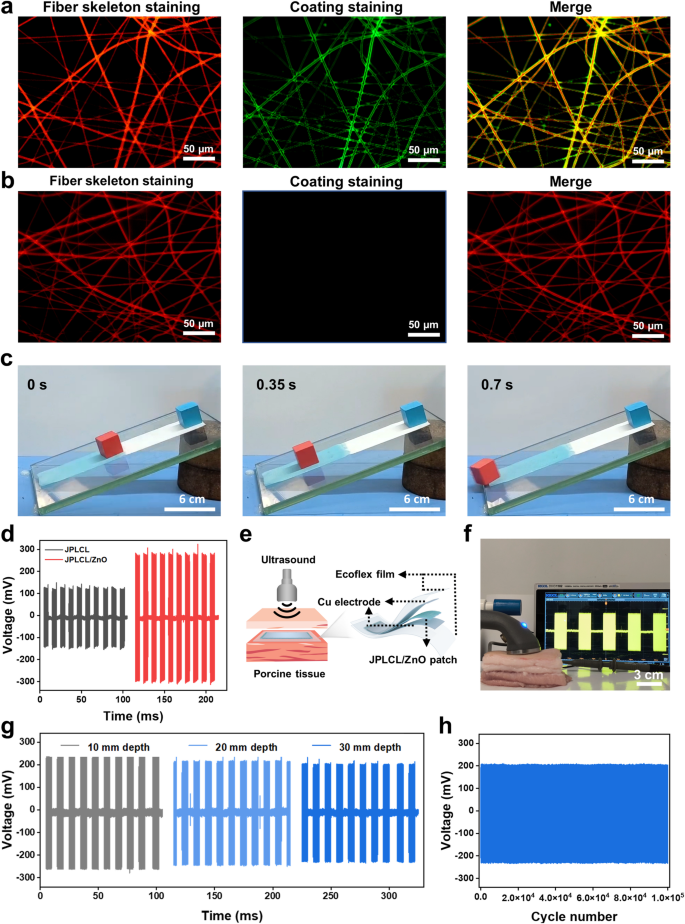
The Janus construction of our JPLCL/ZnO patches was characterised utilizing X-ray photoelectron spectroscopy (XPS) and water contact angle measurements. XPS spectra verify the presence of P and N parts on the highest floor of JPLCL/ZnO patch (Determine S9), indicating the profitable formation of PMPC. As proven in Fig. 2j and okay, the water contact angle is nearly 0° on the highest floor and 122° on the underside floor of JPLCL/ZnO patch, indicating the superhydrophilic nature of PMPC coating. This extremely hydrated floor can successfully resist protein adsorption and mobile attachment, thereby stopping tissue adhesion in defect restore [44, 45]. Furthermore, JPLCL/ZnO patch displays a fibrous morphology with a tough floor, as indicated by the C, O, and P parts predominantly distributed alongside the fibers (Determine S10), indicating that PMPC coating doesn’t disrupt the unique fibrous morphology of JPLCL/ZnO patch. In distinction, we ready a PLCL/ZnO patch with Janus construction utilizing conventional benzophenone therapy (BPLCL/ZnO). It may be clearly seen from the SEM picture (Determine S11) that the unique fibrous morphology of BPLCL/ZnO patches is roofed by a steady PMPC coating, with C, O, and P parts uniformly distributed throughout the whole floor. Conventional benzophenone therapy may destroy the extremely oriented morphology and mechanical anisotropy of the patches [44], resulting in a mechanical mismatch with the belly wall and hindering the restore course of. To raised visualize the spatial relationship between fibers and coatings, JPLCL/ZnO patch was stained with Nile pink for fiber skeleton and sodium fluorescein for PMPC coating, and subsequently noticed beneath a fluorescence microscope. Purple-stained fiber skeleton and green-stained coating are clearly seen on the highest floor of JPLCL/ZnO patch, with the green-stained coating seamlessly conforming to the red-stained fiber skeleton (Fig. 3a), demonstrating that PMPC coating is shaped on every fiber of the highest floor. In distinction, solely the red-stained fiber skeleton is noticed on the underside floor (Fig. 3b), additional confirming the Janus construction of JPLCL/ZnO patch. As a conceptual characterization, we performed a slope slide experiment by sliding two ceramic blocks over each surfaces of JPLCL/ZnO patch. The highest floor of JPLCL/ZnO patch was stained blue with a 0.01% (w/v) erioglaucine disodium salt answer (Good Blue FCF, an FDA-approved biocompatible dye [46, 47]) to tell apart it from the white backside floor. As proven in Fig. 3c, the pink ceramic block strikes easily on the highest floor, whereas no seen motion of the blue ceramic block is noticed on the underside floor (Video S1), indicating the superb lubricating properties of PMPC coating. These outcomes display that JPLCL/ZnO patch with a Janus construction has been efficiently fabricated by establishing PMPC coating on every fiber on its prime floor.
a) Schematic diagram of muscle fibers alignment in human belly wall: (i) The transversus abdominis (higher layer) displays muscle fibers aligned at 0°, whereas the interior indirect (decrease layer) displays muscle fibers aligned at 45°. (ii) The interior indirect (higher layer) displays muscle fibers aligned at 45°, whereas the exterior indirect (decrease layer) displays muscle fibers aligned at 135°. b, c) SEM photos of a(i) area biomimetic JPLCL/ZnO patch (b) and a(ii) area biomimetic JPLCL/ZnO patch (c). d) Digital picture of large-scale JPLCL/ZnO patch. e) Schematic illustration of JPLCL/ZnO patches with completely different orientation stacking ratios. f) Quantitative evaluation of anisotropy ratio, tensile energy, and elastic modulus of JPLCL/ZnO patches with completely different orientation stacking ratios. g) Digital pictures of JPLCL/ZnO patch loaded with a 200 g weight within the parallel and perpendicular instructions. h, i) SEM photos of the highest (h) and backside (i) surfaces of JPLCL/ZnO patch. j, okay) Water contact angles of the highest (j) and backside (okay) surfaces of JPLCL/ZnO patch
Wi-fi electrostimulation remedy, resembling ultrasound remedy, has proven nice potential in selling tissue restore as a result of its non-invasive nature and exact focusing on functionality [48, 49]. Piezoelectric supplies might be wirelessly activated by ultrasound to supply localized electrical stimulation to advertise cell habits and tissue regeneration [50, 51]. The electrical performances of JPLCL/ZnO patches with completely different ZnO contents had been evaluated beneath ultrasound stimulation at an influence depth of 0.5 W cm−2 and a pulse responsibility of fifty%. As proven in Determine S12, the output voltage of JPLCL/ZnO patch will increase progressively with ZnO content material. In distinction to JPLCL patch, JPLCL/ZnO patch displays an ~ 2.4-fold improve in output voltage (Fig. 3d), demonstrating the improved electrical efficiency with the incorporation of ZnO nanoparticles. Below ultrasound stimulation, our patch undergoes cyclic deformation that induces electrical polarization and produces localized electrical alerts, which have been proven to manage mobile behaviors resembling cell migration and proliferation [52,53,54]. Contemplating that the thickness of human belly wall usually ranges from 10 to 30 mm [55], an ex vivo implantation mannequin was designed to simulate its medical utility. As proven in Fig. 3e, JPLCL/ZnO patch was encapsulated between two copper (Cu) electrodes and Ecoflex movies to assemble a versatile system, which was then implanted at completely different depths of porcine tissue. An ultrasound probe was used to ship ultrasound stimulation by the porcine pores and skin, and the output voltages of JPLCL/ZnO patch had been measured and recorded utilizing an oscilloscope (Fig. 3f). Because the implantation depth in porcine tissue will increase from 10 mm to 30 mm, the output voltages of JPLCL/ZnO patch present a slight lower (Fig. 3g and Determine S13). Furthermore, our JPLCL/ZnO patch maintains secure electrical output even at a depth of 30 mm, with no vital voltage variations between the preliminary and last cycles over 105 cycles (Fig. 3h), indicating that ultrasound remedy can successfully penetrate tissues and exactly give attention to focused areas in a non-invasive method. Due to this fact, our JPLCL/ZnO patch might be wirelessly activated by ultrasound to generate localized electrical stimulation for defect restore.
a, b) Fluorescence microscopy photos of the highest (a) and backside (b) surfaces of JPLCL/ZnO patch. c) Digital pictures of the slope slide experiment by sliding two ceramic blocks on the highest (blue shade) and backside (white shade) surfaces of JPLCL/ZnO patch. d) Output voltages of JPLCL and JPLCL/ZnO patches beneath ultrasound stimulation. e, f) Schematic illustration (e) and digital picture (f) of voltage technology of JPLCL/ZnO patch in porcine tissue beneath ultrasound stimulation. g) Output voltages of JPLCL/ZnO patch implanted at completely different depths of porcine tissue. h) Cyclic stability of the output voltage of JPLCL/ZnO patch implanted at a 30 mm depth of porcine tissue throughout 105 cycles
Good biocompatibility and antibacterial properties are important for implanted supplies in tissue restore to make sure each biosafety and stop an infection [56,57,58,59]. The biocompatibility of JPLCL/ZnO patch was evaluated utilizing dwell/useless staining and cell counting kit-8 (CCK-8) assays. The morphologies and cell density of the JPLCL/ZnO group are much like these of the management group (Determine S14a). As well as, the quantitative evaluation of CCK-8 assay exhibits no vital statistical distinction in cell proliferation between the 2 teams (Determine S14b), demonstrating the nice biocompatibility of JPLCL/ZnO patch. In vivo biocompatibility was additional evaluated by implanting PCO and JPLCL/ZnO patches subcutaneously within the dorsal area of rats for five days, after which tissue samples had been collected for HE and immunohistochemical staining, together with CD68 (a macrophage marker) and IL-6 (an inflammatory issue). The JPLCL/ZnO group exhibits a decrease inflammatory response in comparison with the PCO group, and the expressions of CD68 and IL-6 within the JPLCL/ZnO group are considerably decrease than these within the PCO group (Determine S15), indicating that JPLCL/ZnO patch doesn’t induce apparent inflammatory response. To additional consider the in vivo biosafety of the patches beneath ultrasound stimulation, main organs (e.g., coronary heart, liver, spleen, lung, and kidney) had been collected for HE staining after rats had been sacrificed. As proven in Determine S16, no evident tissue injury is noticed within the organs, indicating that our patch beneath ultrasound remedy displays good biosafety in vivo. As well as, the antibacterial properties of JPLCL/ZnO patch towards E. coli and S. aureus had been evaluated utilizing the agar plate incubation technique. The JPLCL/ZnO+US group exhibits fewer colony-forming models (CFU) of E. coli and S. aureus in comparison with the management and JPLCL/ZnO teams (Determine S17a). JPLCL/ZnO patch exhibits bacteriostatic charges of 88% and 86% towards E. coli and S. aureus, respectively, that are additional enhanced to 94% and 93% beneath ultrasound stimulation (Figures S17b and S17c), suggesting a synergistic antibacterial impact between the patch and ultrasound remedy. These outcomes display that JPLCL/ZnO patch displays good biocompatibility and antibacterial properties.
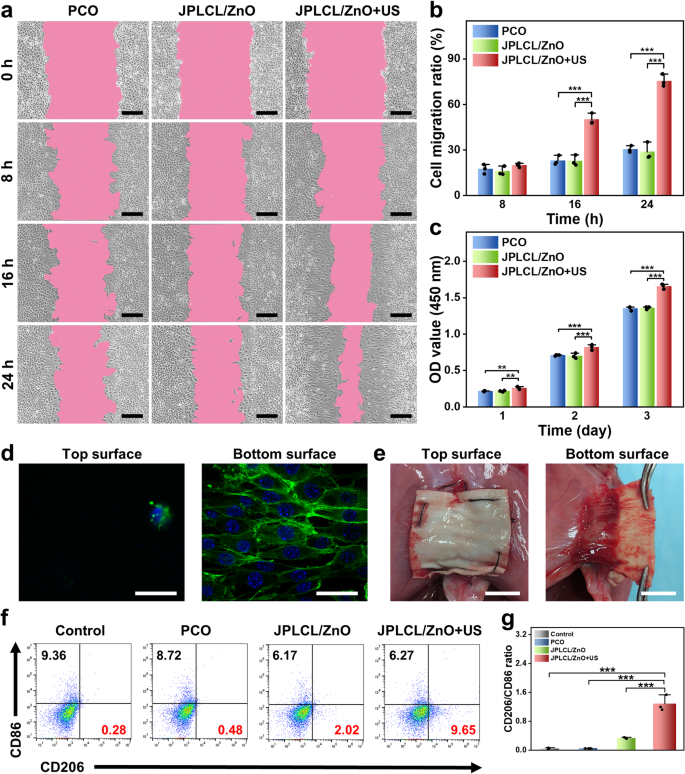
The migration and proliferation of fibroblasts are essential indicators within the tissue restore course of, as they produce numerous extracellular matrix parts and cytokines to advertise tissue regeneration [60,61,62,63]. To research the exercise of fibroblasts cultured with the patch beneath ultrasound stimulation, cell migration and proliferation charges had been evaluated by cell scratch take a look at and cell proliferation assay. The in vitro cell scratch take a look at outcomes present that the JPLCL/ZnO+US group considerably promotes the migration of fibroblasts in comparison with the management and JPLCL/ZnO teams (Fig. 4a and b), which might be attributed to environment friendly guiding impact of electrical fields beneath ultrasound remedy. Cell proliferation charge was quantitatively evaluated utilizing CCK-8 assay. L929 fibroblasts within the JPLCL/ZnO+US group exhibit the next cell depend than these within the management and JPLCL/ZnO teams (Fig. 4c), demonstrating its capability to advertise cell proliferation. Along with selling cell migration and proliferation, belly wall defect restore supplies additionally want uneven regulation of cells to realize anti-adhesion efficiency. Due to this fact, the cell adhesion behaviors of JPLCL/ZnO patches had been assessed to guage the impact of the Janus construction on organic adhesion. L929 fibroblasts had been seeded on each the highest and backside surfaces of JPLCL/ZnO patch and cultured for at some point to seize the fluorescence staining photos. As proven in Fig. 4d, extra L929 fibroblasts adhere to the underside floor of JPLCL/ZnO patch and develop alongside the anisotropic fibers, whereas solely few L929 fibroblasts are noticed on the highest floor, indicating that our JPLCL/ZnO patch can promote directional cell progress whereas stopping tissue adhesion. In distinction, a random JPLCL/ZnO patch was fabricated at a drum rotation pace of 100 rpm, and L929 fibroblasts cultured on these random fibers exhibit multidirectional progress and attachment as a result of lack of anisotropic topology (Determine S18). The bioadhesion habits of JPLCL/ZnO patch was additional evaluated in vivo utilizing a rat belly wall defect mannequin. After 14 days of implantation, the underside floor of JPLCL/ZnO patch adheres properly to the defect tissue, whereas its prime floor can successfully stop visceral adhesion (Fig. 4e). These outcomes display that our JPLCL/ZnO patch can’t solely promote cell migration and proliferation, but in addition stop visceral adhesion.
Apart from cell migration and proliferation, macrophage polarization performs an important function in tissue restore by regulating irritation and selling therapeutic [64,65,66]. To research the impact of JPLCL/ZnO patch on immunoregulation, the expression of CD86 (a marker of M1 macrophages) and CD206 (a marker of M2 macrophages) had been evaluated utilizing movement cytometry. As proven in Fig. 4f and g, the JPLCL/ZnO+US group displays the very best M2-like/M1-like macrophage ratio amongst all of the teams, indicating that JPLCL/ZnO patch beneath ultrasound remedy can improve M2 macrophage polarization.
a) Cell migration photos of fibroblasts for 0, 8, 16, and 24 h within the PCO, JPLCL/ZnO, and JPLCL/ZnO + US teams (scale bars: 200 μm). b) Quantitative evaluation of cell migration (n = 3 impartial samples; ANOVA adopted by Tukey’s a number of comparisons; *** adjusted P < 0.001; error bars = SD; knowledge are introduced as imply ± SD). c) CCK-8 assay of L929 fibroblasts cultured after 1, 2, and three days within the PCO, JPLCL/ZnO, and JPLCL/ZnO + US teams (n = 3 impartial samples; ANOVA adopted by Tukey’s a number of comparisons; ** adjusted P < 0.01; *** adjusted P < 0.001; error bars = SD; knowledge are introduced as imply ± SD). d) Fluorescence photos of L929 fibroblasts cultured on the highest and backside surfaces of JPLCL/ZnO patch for 1 day (scale bars: 40 μm). e) Digital pictures of sentimental tissue adhering to JPLCL/ZnO patch in repairing a rat belly wall defect on the 14th day after surgical procedure (scale bars: 1 cm). f) Quantification of CD206 and CD86 on RAW 264.7 cells by movement cytometry. g) Quantitative evaluation of CD206/CD86 ratio (n = 3 impartial samples; ANOVA adopted by Tukey’s a number of comparisons; *** adjusted P < 0.001; error bars = SD; knowledge are introduced as imply ± SD)
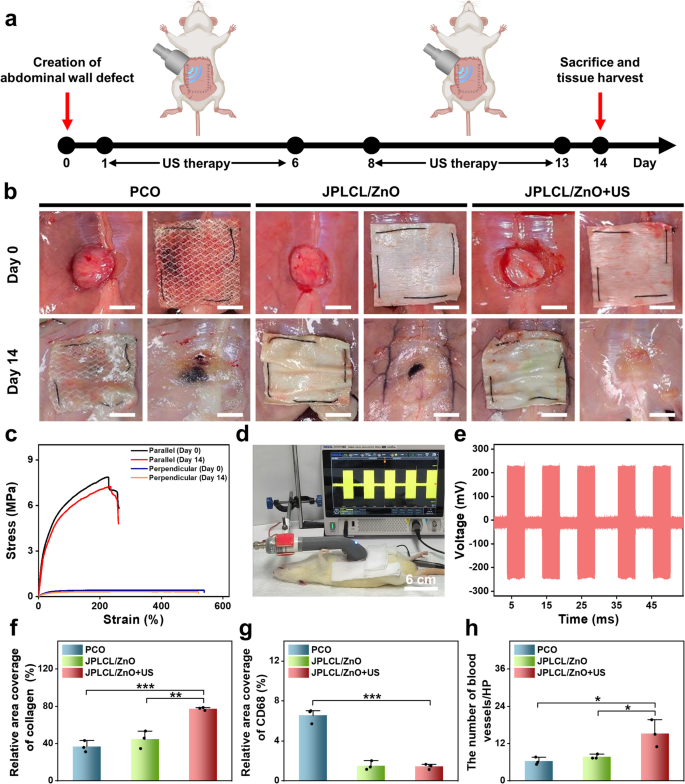
To judge the in vivo pro-healing and anti-adhesion properties of JPLCL/ZnO patch, a rat belly wall defect mannequin was established by making a 15 mm round defect within the belly wall utilizing a round punch. On the 14th day post-surgery, rats had been sacrificed to watch defect restore and adhesion formation (Fig. 5a). Consultant pictures of wound therapeutic and visceral adhesion are proven in Fig. 5b, together with the surgically created defect and sutured patch on day 0, and the implanted patch in situ and the defect web site after patch removing on day 14. JPLCL/ZnO patch doesn’t trigger extreme visceral adhesion, and its anti-adhesion impact is corresponding to that of medical PCO patch. After eradicating the patches to show the defects, the JPLCL/ZnO+US group displays markedly elevated therapeutic in contrast with the opposite teams (Fig. 5b). To judge the mechanical properties of JPLCL/ZnO patch in vivo, stress-strain curves had been analyzed earlier than implantation and 14 days after implantation. JPLCL/ZnO patch exhibits glorious mechanical anisotropy on the 14th day (Fig. 5c), which is useful for defect restore and long-term implantation [31, 67, 68]. As well as, an in vivo electrical efficiency experiment was performed to analyze the feasibility of voltage technology from the patch beneath ultrasound stimulation in a rat mannequin. After anesthetizing the rat with 3% pentobarbital sodium by way of intraperitoneal injection, JPLCL/ZnO patch was implanted within the belly wall defect after which uncovered to ultrasound waves, the output voltage was recorded utilizing an oscilloscope (Fig. 5d and Video S2). Below an ultrasound energy depth of 0.5 W cm−2, our JPLCL/ZnO patch can generate an output voltage of 231 mV (Fig. 5e), confirming the feasibility of delivering electrical stimulation to the defect beneath ultrasound remedy.
To additional consider the pro-healing properties of JPLCL/ZnO patches, tissue samples had been collected from the rat belly wall defects on the 14th day after surgical procedure for histological evaluation. As proven in Determine S19, HE staining outcomes point out that each the management and US teams exhibit incomplete therapeutic, indicating that ultrasound remedy alone hardly promotes wound therapeutic. In distinction to the PCO and JPLCL/ZnO teams, the JPLCL/ZnO + US group exhibits decreased infiltration of inflammatory cells and denser collagen bundles (Figures S20 and S21). Quantitative evaluation reveals that the collagen density within the JPLCL/ZnO+US group (77.4%) is considerably larger than the PCO (36.8%) and JPLCL/ZnO (44.7%) teams (Fig. 5f), indicating its glorious pro-healing property. Furthermore, immunohistochemical staining outcomes reveal that the expression of CD68 is considerably decrease in each the JPLCL/ZnO and JPLCL/ZnO+US teams in comparison with the PCO group (Fig. 5g and Determine S22). Double immunofluorescence staining for CD31 and α-SMA was carried out to guage angiogenesis. CD31 serves as a marker of endothelial cells and displays new blood vessel formation, whereas α-SMA is a marker of vascular clean muscle cells and signifies vessel maturation. The expression ranges of CD31 and α-SMA within the JPLCL/ZnO+US group are considerably larger than these within the PCO and JPLCL/ZnO teams (Fig. 5h and Determine S23), indicating that JPLCL/ZnO patch can promote vascular proliferation beneath ultrasound stimulation. These outcomes present dependable histological proof supporting the pro-healing capability of our JPLCL/ZnO patch beneath ultrasound remedy.
a) Schematic diagram of the restore of belly wall defect in a rat mannequin. b) Digital pictures of wound therapeutic and visceral adhesions formation of belly wall defects within the PCO, JPLCL/ZnO, and JPLCL/ZnO + US teams. Day 0: surgically created defect (left) and sutured patch overlaying the defect (proper). Day 14: implanted patch in situ (left) and defect web site after patch removing (proper) (scale bars: 1 cm). c) Mechanical properties of JPLCL/ZnO patches within the parallel and perpendicular instructions earlier than implantation and after 14 days of in vivo implantation. d) Digital picture of voltage technology from JPLCL/ZnO patch beneath ultrasound stimulation in a rat mannequin. e) Output voltage of JPLCL/ZnO patch beneath ultrasound stimulation. f) Quantitative evaluation of collagen density (n = 3 impartial samples; ANOVA adopted by Tukey’s a number of comparisons; ** adjusted P = 0.002; *** adjusted P < 0.001; error bars = SD; knowledge are introduced as imply ± SD). g) Quantitative evaluation of CD68 (n = 3 impartial samples; ANOVA adopted by Tukey’s a number of comparisons; *** adjusted P < 0.001; error bars = SD; knowledge are introduced as imply ± SD). h) Quantitative evaluation of CD31/α-SMA (n = 3 impartial samples; ANOVA adopted by Tukey’s a number of comparisons; PCO vs. JPLCL/ZnO + US * adjusted P = 0.014; JPLCL/ZnO vs. JPLCL/ZnO + US * adjusted P = 0.031; error bars = SD; knowledge are introduced as imply ± SD)
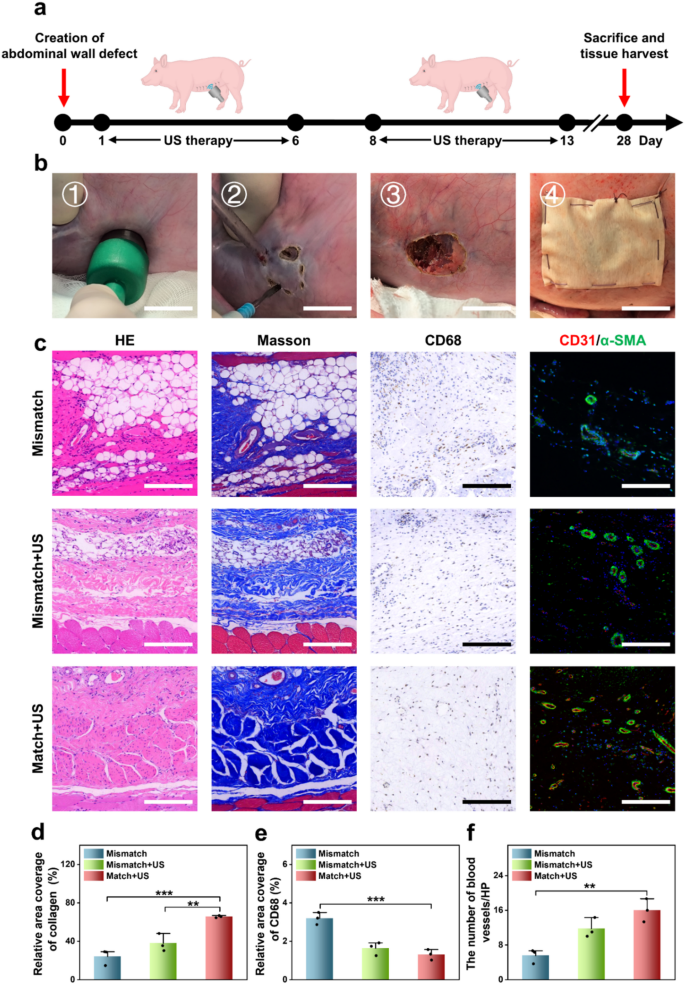
To simulate a clinically related belly wall defect in human, a porcine belly wall defect mannequin was established by making a 2 cm round defect within the belly wall utilizing an ultrasonic scalpel. These defects had been subsequently repaired utilizing patches, which had been secured with 4-0 silk braided sutures (Fig. 6a and b). The experimental therapies had been divided into three teams: the Mismatch group receiving a JPLCL/ZnO patch that misaligned with the pure belly wall anisotropy route, the Mismatch+US group receiving a JPLCL/ZnO patch misaligned with the pure belly wall anisotropy route and ultrasound remedy, and the Match+US group receiving a JPLCL/ZnO patch aligned with the pure belly wall anisotropy route and ultrasound remedy. For the Mismatch+US and Match+US teams, ultrasound therapy was administered at 0.5 W cm−2 for 10 min per day and utilized six days per week. After 28 days, the pigs had been euthanized and tissue samples had been harvested for histological evaluation. HE and Masson staining outcomes point out that the Match+US group displays superior tissue alignment and elevated collagen deposition in comparison with the Mismatch and Mismatch+US teams (Fig. 6c and d). This implies that the biomechanical matching and ultrasound remedy can synergistically promote the restore of belly wall defects. Immunohistochemical and immunofluorescence staining additional reveal that the Match + US group exhibits decrease expression of CD68 and better expression of CD31 and α-SMA in comparison with the Mismatch and Mismatch+US teams (Fig. 6c and e, and 6f). These outcomes display that our JPLCL/ZnO patch can successfully promote tissue alignment, collagen deposition, and vascular proliferation beneath ultrasound remedy. Due to this fact, our JPLCL/ZnO patch not solely prompts electrical stimulation to advertise tissue restore beneath ultrasound remedy, but in addition integrates glorious mechanical anisotropy and anti-adhesion properties for the environment friendly restore of belly wall defects.
a, b) Schematic diagram (a) and surgical course of (b) of the restore of belly wall defect in a porcine mannequin (scale bars: 2 cm). c) Photographs of HE staining, Masson staining, immunohistochemical staining, and immunofluorescence staining for the Mismatch, Mismatch+US, and Match+US teams (scale bars: 200 μm) d) Quantitative evaluation of collagen density (n = 3 impartial samples; ANOVA adopted by Tukey’s a number of comparisons; ** adjusted P = 0.008; *** adjusted P < 0.001; error bars = SD; knowledge are introduced as imply ± SD). e) Quantitative evaluation of CD68 (n = 3 impartial samples; ANOVA adopted by Tukey’s a number of comparisons; *** adjusted P < 0.001; error bars = SD; knowledge are introduced as imply ± SD). f) Quantitative evaluation of CD31/α-SMA (n = 3 impartial samples; ANOVA adopted by Tukey’s a number of comparisons; ** adjusted P =0.003; error bars = SD; knowledge are introduced as imply ± SD)