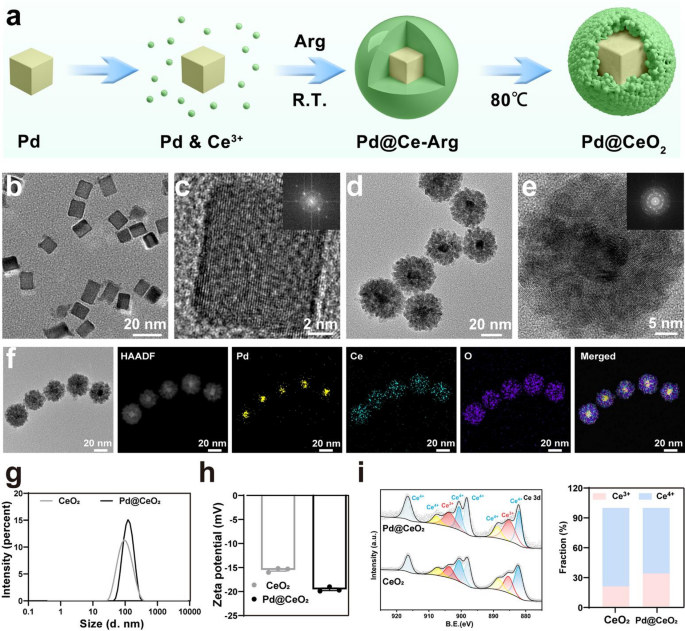
Morphological and structural evaluation of Pd@CeO2
Pd@CeO2 nanozymes had been synthesized by way of a multi-step course of depicted in Fig. 1a. Uniform Pd nanocubes (~ 18 nm) had been initially shaped by lowering Na2PdCl4 utilizing ascorbic acid, polyvinylpyrrolidone, and potassium bromide, guaranteeing monodispersed and exactly sized nanoparticles (Fig. 1b–c). Subsequently, cerium nitrate was launched within the presence of L-Arginine as a capping agent, facilitating CeO2 nanoparticles’ self-assembly onto the Pd nanocube surfaces. This course of resulted in a definite core-shell Pd@CeO2 construction, confirmed by TEM imaging exhibiting full and uniform encapsulation of Pd cores by CeO2 shells (Fig. 1d–e). Power-dispersive X-ray spectroscopy (EDS) elemental mapping (Fig. 1f) demonstrated the spatial distribution of Pd, Ce, and O, additional confirming profitable core-shell formation. The crystallinity of Pd and Pd@CeO2 phases was validated via X-ray diffraction (XRD), which exhibited attribute peaks similar to each supplies (Determine S1). Dynamic gentle scattering (DLS) measurements confirmed that Pd@CeO2 nanozymes exhibited a imply particle diameter of 103.9 ± 0.56 nm (Fig. 1g), whereas the zeta potential measurement indicated a floor cost of −19.5 ± 0.47 mV (Fig. 1h).
To elucidate the electron-injection mechanism, X-ray photoelectron spectroscopy (XPS) was employed. Evaluation of the Ce 3 d spectra revealed a marked improve within the Ce3+/Ce4+ ratio in Pd@CeO2 (34.23%/65.77%) in contrast with pristine CeO2 (21.29%/78.71%). Moreover, peak becoming of the Ce 3d spectra revealed that Pd@CeO2 displays general decrease binding energies than CeO2 (Fig. 1i). Specifically, the improved depth of Ce3+ peaks at these shifted positions signifies electron enrichment on the CeO2 interface, arising from interfacial electron switch from Pd to CeO2, which supplies strong ROS-scavenging efficiency. Stability assessments over 7 days revealed minimal particle aggregation or cost alterations, underscoring the nanoparticle’s suitability for physiological purposes (Determine S2).
Synthesis and characterization of Pd@CeO2 nanozymes. (a) Schematic illustration of the Pd@CeO2 nanozymes preparation course of. (b) TEM pictures of Pd nanocubes. (c) Electron diffraction (SAED) sample of Pd nanocubes. (d) TEM pictures of Pd@CeO2 nanozymes. (e) SAED sample of Pd@CeO2 nanozymes. (f) Aspect mapping of Pd, Ce, O, and the merged picture of those three parts. (g–h) Particle measurement distribution and zeta potential of Pd@CeO2 nanozymes. (i) XPS of Pd@CeO2 nanozymes
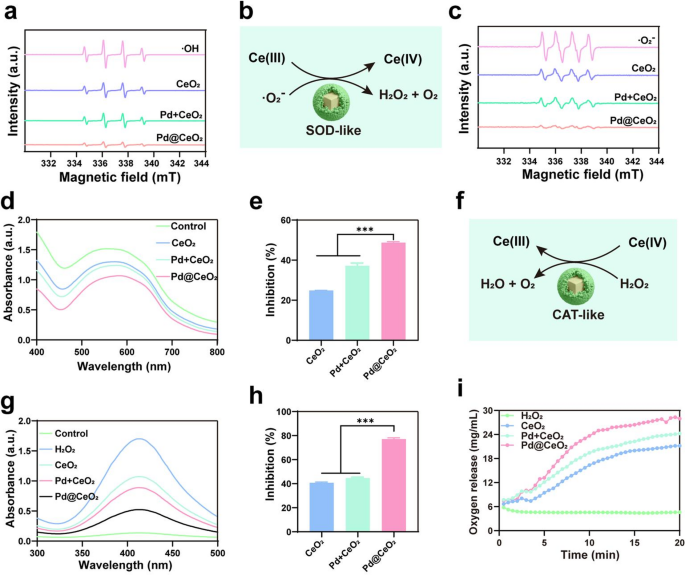
Enzyme-mimicking actions of Pd@CeO2 and mechanistic insights from DFT calculations
ROS together with hydroxyl radicals (·OH), superoxide anions (·O2⁻), and hydrogen peroxide (H2O2), are pivotal in quite a few organic and pathological processes [31, 32]. We first evaluated the ·OH scavenging functionality of Pd@CeO2 utilizing electron spin resonance (ESR) spectroscopy. ESR spectra (Fig. 2a) displayed a definite 1:2:2:1 quartet sign attribute of DMPO-·OH adducts generated via the Fenton response. In comparison with controls, Pd@CeO2 exhibited considerably lowered ESR sign depth, demonstrating superior ·OH scavenging because of the enhanced catalytic impact of Pd@CeO2. Subsequently, the SOD-like exercise of Pd@CeO2 was investigated. ESR spectra of ·O2⁻ (Fig. 2c) revealed a pronounced discount within the typical 1:1:1:1 quartet upon remedy with Pd@CeO2, confirming environment friendly superoxide neutralization. Quantitative evaluation utilizing the nitro blue tetrazolium (NBT) assay (Fig. 2d–e) additional validated this enhanced efficiency, as Pd@CeO2 exhibited the bottom absorbance at 560 nm, reflecting the very best inhibition charge (48.72%) in contrast with CeO2 (24.81%) and Pd + CeO2 mixtures (37.18%). CAT-like exercise, important for H2O2 cleansing, was evaluated utilizing a colorimetric assay (Fig. 2g–h). Pd@CeO2 confirmed the bottom absorbance at 405 nm and a markedly increased H2O2 inhibition charge (77.1%) in comparison with CeO2 (40.8%) and Pd + CeO2 (44.7%). Moreover, dissolved oxygen measurements revealed speedy catalytic decomposition of H2O2 to O2 upon Pd@CeO2 remedy, rising O2 concentrations from 7.5 µg/mL to twenty-eight.3 µg/mL inside 20 min—considerably outperforming CeO2 nanoparticles (21.2 µg/mL) (Fig. 2i). These outcomes exhibit that Pd@CeO2 displays complete ROS scavenging capability, integrating ·OH, ·O2⁻, and H2O2 elimination via synergistic multi-enzyme–like actions.
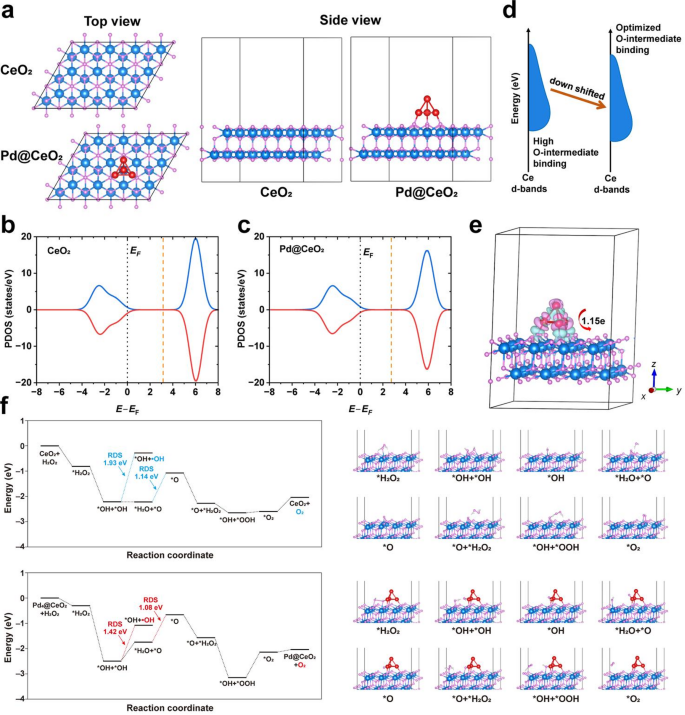
To elucidate the mechanism underlying the distinctive catalytic efficiency of the CeO2 (111) slab adorned with Pd clusters, density useful principle (DFT) calculations had been carried out. A Pd@CeO2 mannequin was constructed and in contrast with the pristine CeO2 slab (Fig. 3a). Owing to the pronounced empty-band distribution in CeO2, the place of the d-band heart relative to the Fermi degree was fastidiously thought-about. For the CeO2 (111) slab, the d-band heart is situated at 3.13 eV (Fig. 3b). Upon adsorption of a Pd cluster, the d-band heart shifts downward by 0.38 eV to 2.75 eV (Fig. 3c), indicating a weakened adsorption power of oxygen intermediates on the floor Ce websites. These outcomes exhibit that the incorporation of Pd clusters induces pronounced digital interactions inside the catalyst, leading to a downward shift of the d-band heart. This adjustment optimizes the adsorption of important intermediates and thereby enhances catalytic effectivity, as illustrated in Fig. 3d. The cost density distinction evaluation additional confirms the digital modulation induced by the Pd clusters. As proven in Fig. 3e, adsorption of a Pd cluster ends in a cost switch of roughly 1.15 electrons from the cluster to the CeO2 substrate, as decided by Bader cost evaluation. This electron donation will increase the electron density on the substrate, strengthening digital interactions on CeO2. This remark is per the projected density of states (PDOS) evaluation, which reveals the downward shift of the d-band heart. Moreover, the adsorption energies of oxygen intermediates on the Pd@CeO2 floor had been systematically evaluated. The response vitality limitations of every elementary step had been additionally calculated, revealing a transparent relationship between the overlap of transition-metal d orbitals and the adsorption of oxygen intermediates on the catalyst floor (Fig. 3f). For each CeO2 and Pd@CeO2 methods, we discovered that the rate-determining step (RDS) for H2O2 decomposition producing ·OH radicals (i.e., the peroxidase-like response) is related to increased vitality limitations in comparison with H2O2 decomposition producing O2 (i.e., the CAT-like response), with values of 1.93 eV vs. 1.14 eV and 1.42 eV vs. 1.08 eV, respectively. This end result means that the CAT-like pathway predominates within the general response. Notably, the adsorption of Pd clusters considerably reduces the RDS. Particularly, the formation of *O is recognized because the RDS for Pd@CeO2, with a calculated vitality barrier of 1.08 eV, which is decrease than the 1.14 eV barrier noticed within the pristine CeO2 system. This discount will be attributed to the improved electron density on the Ce websites induced by Pd cluster adsorption, which is per the Bader cost evaluation, and facilitates H2O2 discount and consequently promotes O2 evolution, thereby conferring superior CAT-like exercise. These findings present elementary insights into how Pd clusters regulate the digital construction of CeO2, thereby enhancing catalytic exercise.
ROS elimination by Pd@CeO2 nanozymes. (a) ESR spectra of ·OH radicals trapped by DMPO in numerous samples (optimistic group, CeO2, Pd + CeO2 and Pd@CeO2). (b) Schematic illustration of the SOD-like catalytic mechanism of Pd@CeO2. (c) ESR spectra of ·O2− scavenging exercise for CeO2, Pd + CeO2, and Pd@CeO2. (d) NBT assay exhibiting the discount of ·O2− by Pd@CeO2 via decreased absorbance at 560 nm. (e) Inhibition charges of ·O2− by totally different formulations. (f) Schematic of the CAT-like catalytic mechanism of Pd@CeO2. (g) CAT-like exercise of Pd@CeO2 was evaluated utilizing a catalase assay package, the place the lower in absorbance at 405 nm displays H2O2 decomposition. (h) Inhibition charges of H2O2 decomposition by CeO2, Pd + CeO2, and Pd@CeO2. (i) Oxygen era throughout H2O2 decomposition by CeO2 NPs, Pd + CeO2 NPs, and Pd@CeO2
DFT-based mechanistic understanding of Pd@CeO2 catalysis. (a) Fashions of the CeO2 (111) slab (denoted as CeO2) and the CeO2 (111) slab with an adsorbed Pd cluster (denoted as Pd@CeO2). Spheres in teal, crimson and gray characterize cerium, oxygen and palladium, respectively. (b–c) PDOS plots of the (b) CeO2 and (c) Pd@CeO2. (d) Schematic illustration exhibiting that the d-band heart shift of CeO2 induced by Pd4 modulates the binding skill of O-containing intermediates. (e) Cost density distinction distribution of the Pd@CeO2, and insert is Bader cost evaluation of Pd4 on CeO2 (111) slab. The pink and cyan volumes characterize electron depletion and accumulation, respectively. Spheres in teal, crimson and gray characterize cerium, oxygen and palladium, respectively. (f) Power profiles and the corresponding configurations for the formation of floor peroxo species on the CeO2 (111) slab, both pristine (denoted as CeO2) or adorned with a Pd cluster (denoted as Pd@CeO2). The speed-determining step (RDS) is highlighted within the plots. Spheres in blue, white, pink and crimson characterize cerium, hydrogen, oxygen and palladium, respectively
In vitro ROS-scavenging functionality of Pd@CeO2 nanozymes
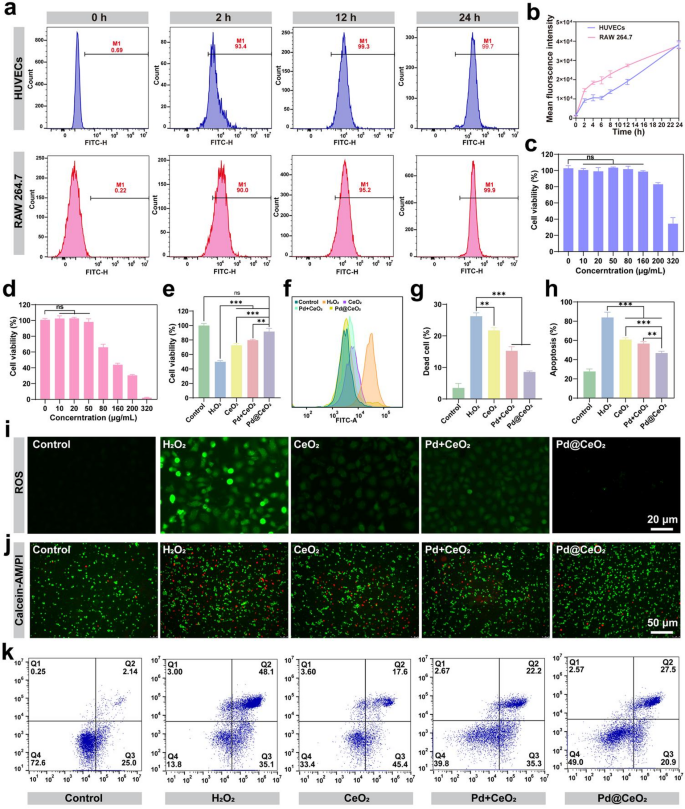
Constructing on the strong enzyme-mimetic catalytic actions of Pd@CeO2, we evaluated their mobile uptake, biocompatibility, and cytoprotective efficiency in opposition to oxidative stress. The internalization of Pd@CeO2 nanozymes was monitored in HUVEC and RAW 264.7 cells utilizing FITC labeling. Fluorescence microscopy and move cytometry persistently demonstrated environment friendly, time-dependent mobile uptake of Pd@CeO2, with uptake effectivity reaching over 95% inside 12 h and approaching full internalization by 24 h (Fig. 4a–b). Biocompatibility evaluation utilizing CCK-8 assays revealed negligible cytotoxicity of Pd@CeO2 at concentrations ≤ 50 µg/mL in each HUVEC and RAW 264.7 cells (Fig. 4c–d). Longitudinal evaluation over a three-day interval confirmed the absence of opposed results on cell proliferation, highlighting the suitability of Pd@CeO2 for biomedical purposes (Determine S3). Subsequent, we evaluated the protecting position of Pd@CeO2 in opposition to H2O2-induced oxidative damage. Pretreatment with Pd@CeO2 markedly enhanced HUVEC viability (91.66%), outperforming CeO2 (72.72%) and Pd + CeO2 mixtures (79.79%) (Fig. 4e). Persistently, intracellular ROS ranges, visualized by DCFH-DA fluorescence, had been considerably diminished within the Pd@CeO2 group, underscoring its superior scavenging efficacy relative to the controls (Fig. 4f and that i and Determine S4–S5). Calcein-AM/PI staining additional confirmed enhanced cell survival and lowered cell demise following Pd@CeO2 remedy beneath oxidative stress situations (Fig. 4g and j). Circulate cytometry analyses utilizing Annexin V-FITC/PI staining demonstrated a considerably decrease apoptosis charge in Pd@CeO2-treated cells, highlighting strong anti-apoptotic results (Fig. 4h and ok). Collectively, the findings set up Pd@CeO2 as a secure and environment friendly nanozymes with superior antioxidant and anti-apoptotic properties, highlighting its promise for biomedical purposes in oxidative stress–pushed pathologies.
Biocompatibility and protecting results of Pd@CeO2 in opposition to oxidative injury. (a) Circulate cytometry evaluation of FITC-labeled Pd@CeO2 uptake by HUVECs and RAW 264.7 macrophages at totally different time factors (0, 2, 12, and 24 h). (b) quantification of move cytometry outcomes. (c) Cytotoxicity of Pd@CeO2 on HUVECs at varied concentrations (0, 10, 20, 50, 80, 160, 200, and 320 µg/mL). (d) Cytotoxicity of Pd@CeO2 on RAW 264.7 macrophages at varied concentrations (0, 10, 20, 50, 80, 160, 200, and 320 µg/mL). (e) Cell viability of CeO2, Pd + CeO2, and Pd@CeO2 (50 µg/mL) in opposition to H2O2-induced oxidative injury. (f) Circulate cytometric evaluation of intracellular ROS ranges in oxidatively pressured HUVEC cells. (g) Statistical evaluation of cell mortality in injured cells. (h) Statistical evaluation of apoptosis charge in every group. (i) DCFH-DA fluorescence microscopy pictures exhibiting ROS ranges in every group. Scale bars: 20 μm. (j) Calcein-AM/PI fluorescence staining of HUVECs handled with H2O2 and or totally different formulations. Scale bars: 50 μm. (ok) Circulate cytometry evaluation of apoptosis charges after totally different therapies
Molecular mechanisms of Pd@CeO2 in suppressing apoptosis and irritation
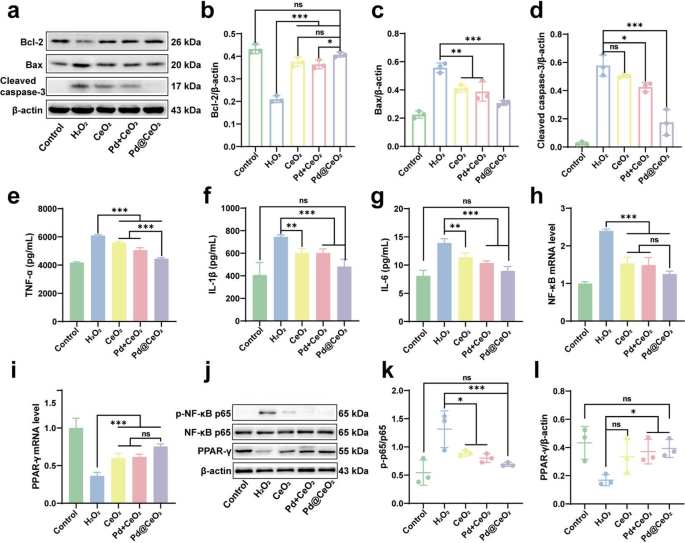
To additional elucidate the protecting mechanisms conferred by Pd@CeO2 nanozymes in opposition to oxidative stress-induced damage, we evaluated their affect on apoptosis-related pathways. Apoptosis, prominently regulated by proteins together with Bcl-2 (anti-apoptotic), Bax (pro-apoptotic), and cleaved Caspase-3 (executioner of apoptosis), is central to I/R damage pathology [33, 34]. Western blot evaluation (Fig. 5a–d) demonstrated that Pd@CeO2 considerably elevated Bcl-2 expression whereas concurrently suppressing Bax and cleaved Caspase-3 ranges in comparison with CeO2 and Pd + CeO2. These findings point out a sturdy inhibition of apoptosis by way of modulation of the Bax/Bcl-2/caspase-3 pathway, underscoring the potent cytoprotective capabilities of Pd@CeO2.
Irritation represents one other pivotal side of I/R damage, which is additional aggravated by extreme ROS era [35, 36]. Using an LPS-stimulated RAW 264.7 macrophage irritation mannequin, ELISA assays revealed that Pd@CeO2 markedly attenuated secretion of key pro-inflammatory cytokines TNF-α, IL-1β, and IL-6 in comparison with the LPS-treated management teams (Fig. 5e–g). This highlights the substantial anti-inflammatory efficacy of Pd@CeO2 in mobile fashions. To uncover the molecular foundation underlying these anti-inflammatory results, quantitative PCR and Western blot analyses assessed the expression of inflammation-related elements NF-κB and PPAR-γ. The LPS-driven improve in NF-κB mRNA expression was considerably suppressed by Pd@CeO2, highlighting its strong capability to modulate this pivotal pro-inflammatory transcription issue (Fig. 5h). Conversely, Pd@CeO2 considerably upregulated PPAR-γ mRNA, supporting its position in resolving irritation (Fig. 5i). Protein-level analyses additional confirmed these outcomes, demonstrating lowered phosphorylation of NF-κB p65 and elevated PPAR-γ protein expression in Pd@CeO2-treated teams in comparison with controls (Fig. 5j–l). These outcomes recommend that Pd@CeO2 modulate inflammatory responses by downregulating the NF-κB pathway and upregulating the anti-inflammatory PPAR-γ pathway.
Anti-apoptotic and anti inflammatory mechanisms of Pd@CeO2. (a) Western blot evaluation of Bcl-2, Bax, and cleaved caspase-3 protein expression ranges. Quantification of (b) Bcl-2, (c) Bax, and (d) caspase-3 protein expression ranges. ELISA outcomes exhibiting (e) TNF-α, (f) IL-1β, and (g) IL-6 secretion ranges after handled with CeO2, Pd + CeO2, or Pd@CeO2. Quantification of (h) NF-κB and (i) PPAR-γ mRNA expression ranges by qPCR. (j) Western blot evaluation of p-NF-κB p65, NF-κB p65, and PPAR-γ protein expression. Quantification of (ok) p-p65 and (l) PPAR-γ protein expression ratio
Restoration of angiogenic capability by Pd@CeO2 in oxidative environments
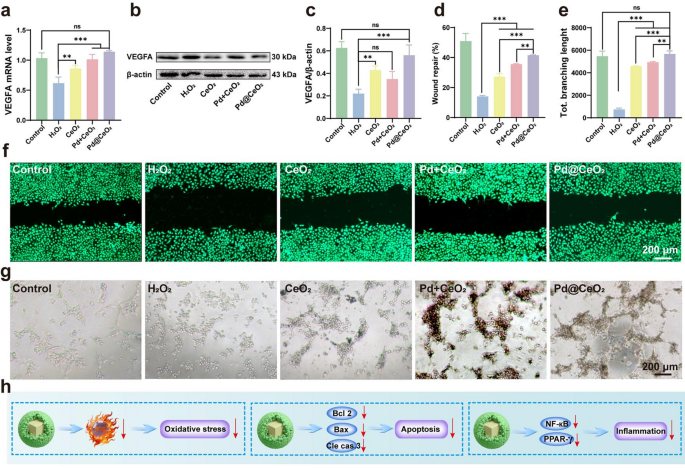
Constructing upon the demonstrated anti-apoptotic and anti inflammatory properties of Pd@CeO2, we additional investigated its angiogenic capability beneath oxidative stress, a crucial think about tissue restore and flap survival. Angiogenesis is notably impaired by I/R damage, thus emphasizing its significance in restoration [37, 38]. qPCR evaluation revealed that Pd@CeO2 remedy markedly upregulated VEGFA mRNA, a key driver of angiogenesis, in H2O2-stimulated HUVECs in contrast with controls, CeO2, and Pd + CeO2 (Fig. 6a). Western blot analyses corroborated this, revealing considerably increased VEGFA protein ranges in Pd@CeO2-treated cells relative to H2O2-exposed cells (Fig. 6b–c). These outcomes spotlight the sturdy capability of Pd@CeO2 to reestablish angiogenic signaling pathways disrupted by oxidative stress. We subsequent evaluated endothelial cell migration utilizing scratch assays. H2O2 publicity markedly impaired migratory capability, whereas remedy with CeO2, Pd + CeO2, and particularly Pd@CeO2 considerably promoted wound closure (Fig. 6d). Experiments additional confirmed that Pd@CeO2 achieved probably the most pronounced enhancement of migration, surpassing all different therapies (Fig. 6f). Lastly, we assessed angiogenic performance by way of endothelial tube formation assays. H2O2-induced oxidative stress severely disrupted tube formation, evident by lowered branching and community complexity. Notably, pretreatment with Pd@CeO2 successfully restored endothelial tube formation, considerably enhancing complete branching size in comparison with CeO2 and Pd + CeO2 therapies ((Fig. 6e and g). These collective outcomes place Pd@CeO2 as a extremely efficient nanozyme, able to considerably selling angiogenesis beneath oxidative stress, thereby highlighting its substantial therapeutic potential in ischemic tissue restore.
In vitro investigations revealed that Pd@CeO2 orchestrates multifaceted cytoprotective mechanisms by lowering oxidative stress, inhibiting apoptosis via the Bcl-2/Bax/caspase axis, and dampening inflammatory cascades mediated by NF-κB and PPAR-γ (Fig. 6h), thereby establishing a coherent mechanistic foundation for its therapeutic potential.
Enhancement of angiogenesis in oxidative stress-damaged HUVECs by Pd@CeO2. (a) qPCR evaluation of VEGFA mRNA expression following remedy with CeO2, Pd + CeO2, or Pd@CeO2. (b) Western blot evaluation of VEGFA protein expression. (c) Quantification of VEGFA protein expression degree. (d) Statistical evaluation of wound restore charge of every group. (e) Statistical analysis of the entire dendritic branching size throughout experimental teams. (f) Consultant pictures of the scratch assay in HUVECs beneath H2O2-induced oxidative stress. Scale bar: 200 μm. (g) Consultant pictures of tube formation assays in HUVECs uncovered to H2O2. Scale bar: 200 μm. (h) Diagrammatic illustration of Pd@CeO2-mediated mechanisms in attenuating oxidative stress, inhibiting apoptosis, and suppressing irritation
Therapeutic efficacy of Pd@CeO2 in a rat pores and skin flap ischemia–reperfusion damage mannequin
To preliminarily assess the in vivo biosafety of Pd@CeO2 nanozymes, we examined their biocompatibility via hemocompatibility and histological research. Hemolysis assays revealed an exceptionally low hemolysis charge (< 10%) throughout concentrations as much as 50 µg/mL, indicating glorious blood compatibility (Determine S6). Blood routine and biochemical analyses carried out 14 days post-injection revealed that main hematological indices, in addition to hepatic and renal perform markers (ALT, AST, BUN, and creatinine), remained inside physiological ranges and had been indistinguishable from these of the management group, thereby substantiating the systemic biosafety of the remedy (Desk S1-S2). Histological examination of main organs (coronary heart, liver, spleen, lung, and kidney) by H&E staining at 7 and 14 days revealed no detectable pathological alterations, thereby reinforcing the superb biosafety profile of Pd@CeO2 (Determine S7).
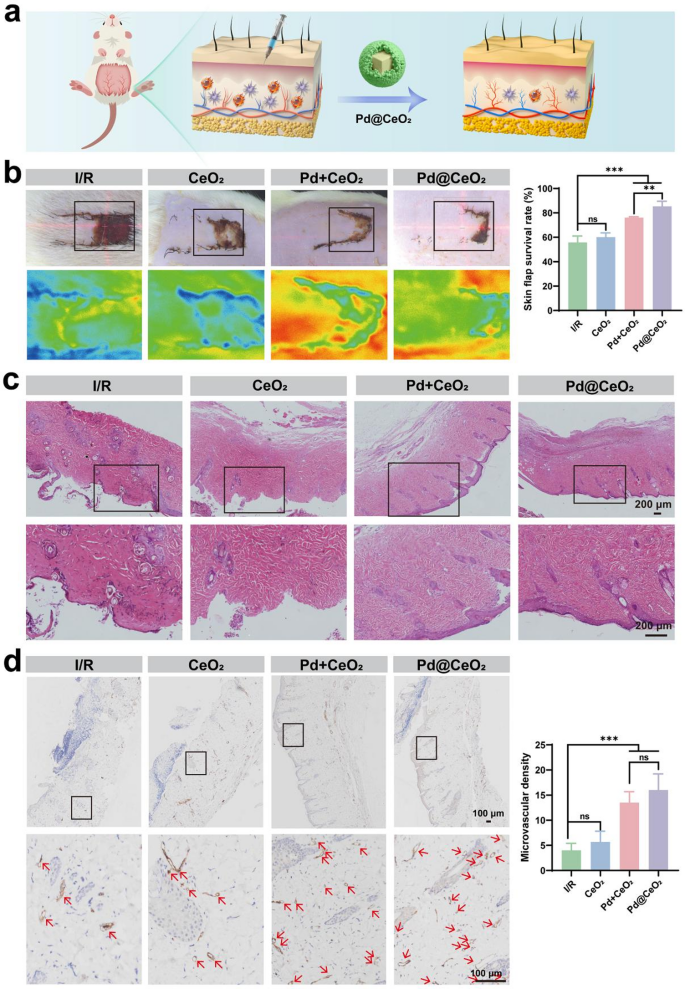
Subsequently, therapeutic efficacy was evaluated utilizing a rat dorsal pores and skin flap I/R damage mannequin. After 6 h of ischemia adopted by reperfusion, in depth flap necrosis was noticed within the untreated controls, whereas Pd@CeO2 remedy markedly preserved tissue integrity and minimized necrotic injury (Fig. 7a-b). Actual-time microcirculation assessed by way of laser speckle imaging revealed considerably enhanced blood perfusion within the Pd@CeO2 group, which achieved the very best flap survival charge (85.42%) in comparison with CeO2 (60.10%), Pd + CeO2 (77.14%), and untreated teams (55.76%) (Fig. 7b). H&E staining additional demonstrated important preservation of tissue structure in Pd@CeO2-treated flaps, characterised by intact epidermal layers, well-maintained hair follicles, and regular dermal collagen deposition, contrasting sharply with extreme epidermal thinning in management teams (Fig. 7c). Given the promising in vitro angiogenic results of Pd@CeO2, we subsequently evaluated its pro-angiogenic capabilities inside an in vivo I/R pores and skin flap mannequin. Immunohistochemical staining for the endothelial marker CD31 was carried out to evaluate neovascularization inside flap tissues. Outcomes indicated compromised angiogenesis within the I/R group, exhibiting microvascular densities of roughly 4.0 vessels per discipline. In distinction, teams handled with Pd + CeO2 and significantly Pd@CeO2 displayed significantly enhanced neovascularization, with microvessel densities of 13.5 and 16.0, respectively (Fig. 7d). These findings underscore the superior efficacy of Pd@CeO2 in selling angiogenesis beneath I/R damage situations.
Analysis of the therapeutic results of Pd@CeO2 in a rat pores and skin flap I/R damage mannequin. (a) Schematic illustration of the therapeutic course of within the I/R damage mannequin. (b) Consultant pictures of pores and skin flaps on day 7 post-surgery for the I/R, CeO2, Pd + CeO2, and Pd@CeO2. (c) HE staining of distal pores and skin flap tissue sections. Scale bars: 200 μm. (d) Consultant pictures of immunohistochemical staining of CD31 in I/R, CeO2, Pd + CeO2, and Pd@CeO2 teams
Mitigation of irritation and apoptosis by Pd@CeO2 in I/R pores and skin flaps
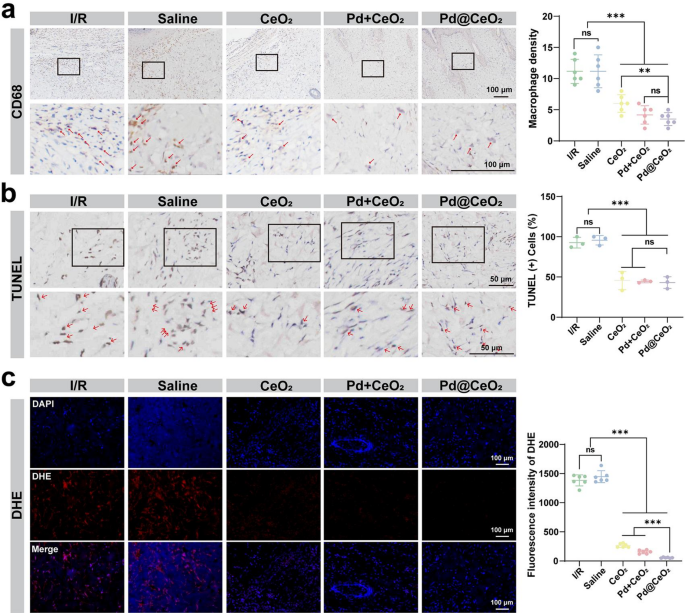
To additional validate the anti-inflammatory potential of Pd@CeO2, immunohistochemical staining of CD68 was carried out to quantify macrophage infiltration, indicative of irritation inside pores and skin flaps. As proven in Fig. 8a, markedly elevated macrophage densities had been detected in each the I/R and saline teams (≈ 11.17 every), reflecting pronounced inflammatory activation. In distinction, macrophage infiltration was considerably lowered within the CeO2 (≈ 6.0) and Pd + CeO2 teams (≈ 4.17), with probably the most pronounced attenuation noticed within the Pd@CeO2-treated group (≈ 3.5), thereby underscoring the potent in vivo anti-inflammatory efficacy of Pd@CeO2. Moreover, TUNEL assays had been employed to guage apoptosis inside the flap tissues. Distinctly increased densities of TUNEL-positive cells had been detected within the I/R and saline management teams, reflecting important apoptotic exercise (apoptosis charges of 92.67% and 95.67%, respectively). Remedy teams confirmed considerably lowered apoptosis charges, with CeO2 at 45.68%, Pd + CeO2 at 44.49%, and notably, Pd@CeO2 demonstrating the bottom apoptosis at 43.08% (Fig. 8b). Lastly, DHE staining indicated that Pd@CeO2 remedy successfully suppressed ROS accumulation in flap tissues, reinforcing the correlation between strong ROS scavenging and improved flap viability and structural integrity (Fig. 8c). These outcomes collectively verify that Pd@CeO2 successfully mitigates apoptosis, doubtlessly by way of strong ROS scavenging and lowered inflammatory signaling, thus underscoring its substantial therapeutic potential in addressing oxidative and inflammatory injury throughout I/R damage.
Anti-inflammatory and anti-apoptotic results of Pd@CeO2 in an I/R skin-flap mannequin. (a) Immunohistochemical staining and semi-quantitative evaluation of CD68 to guage macrophage densities within the pores and skin flap tissues, with crimson arrows indicating macrophages, bar = 100 μm. (b) TUNEL staining and quantitative evaluation to detect apoptotic cells in pores and skin flap tissues, with crimson arrows highlighting apoptotic cells. Scale bars: 50 μm. (c) ROS ranges and semi-quantitative evaluation in pores and skin flap tissues assessed by way of DHE staining. Scale bars: 100 μm
Transcriptomic evaluation of Pd@CeO2-mediated safety in opposition to I/R damage
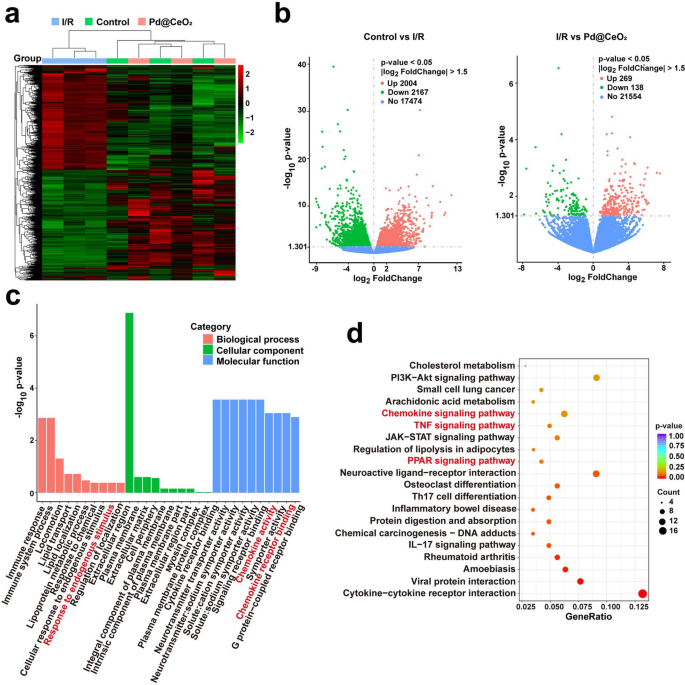
To realize complete insights into the molecular mechanisms underlying the therapeutic actions of Pd@CeO2 in I/R damage, transcriptomic profiling was carried out. Hierarchical clustering of differentially expressed genes (DEGs) revealed distinct transcriptional signatures among the many Management, I/R, and Pd@CeO2-treated teams (Fig. 9a). Volcano plots additional delineated considerably altered gene expressions, highlighting 2004 upregulated and 2167 downregulated genes when evaluating the management and I/R teams. In distinction, Pd@CeO2 remedy induced important expression modifications, with 269 genes upregulated and 138 genes downregulated in comparison with the I/R group (Fig. 9b). Gene Ontology (GO) enrichment evaluation indicated that Pd@CeO2 remedy notably influenced organic processes associated to irritation and immune responses, mobile parts related to membrane and extracellular matrix, and molecular capabilities involving receptor exercise and signaling transduction (Fig. 9c). Notably, enriched organic processes akin to “inflammatory response”, “immune response”, and “mobile response to cytokine stimulus” had been considerably modulated upon Pd@CeO2 remedy, indicating its strong anti-inflammatory potential on the transcriptomic degree. Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway evaluation additional highlighted key signaling pathways considerably regulated by Pd@CeO2 remedy, together with TNF, Chemokine, and PPAR signaling pathways (Fig. 9d). These outcomes corroborate earlier findings concerning the involvement of those pathways in inflammatory modulation and tissue restore, offering strong transcriptomic validation for the anti-inflammatory and regenerative results noticed in earlier mobile and histological assessments.