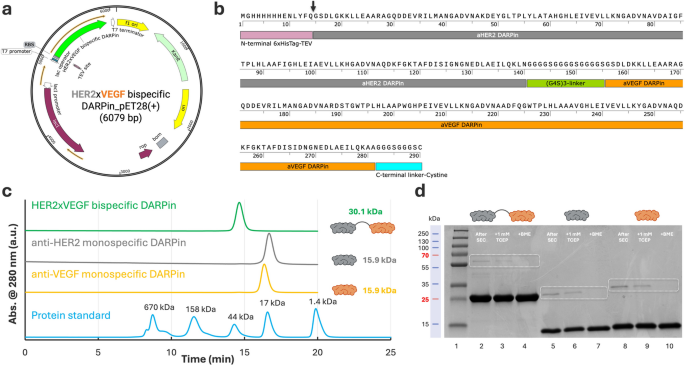
Cloning, expression, purification, and characterization
To allow the manufacturing of a bispecific HER2×VEGF DARPin, we designed a modular assemble by fusing a high-affinity anti-HER2 (area IV) DARPin G3 [21] and an anti-VEGF DARPin Abicipar pegol [23] by means of a versatile (G₄S)₃ linker, incorporating an N-terminal 6×His-tag-TEV (tobacco etch virus) protease cleavage web site and a C-terminal single cysteine residue to facilitate downstream site-specific conjugation (Fig. 1a–b). Each the anti-HER2 DARPin (G3) and anti-VEGF DARPin (Abicipar pegol) are derived from a consensus-designed ankyrin repeat scaffold, initially primarily based on structural motifs present in human ankyrin proteins [21, 25]. This design contributes to their low immunogenicity, favorable folding, and excessive affinity [21, 23, 25, 26]. The bispecific assemble (~ 30 kDa), which contains these two domains, maintains the identical scaffold structure and is predicted to exhibit comparable immunological and biophysical properties. Observe, biparatopic DARPins comparable to 6L1G (i.e., DARPin 9.26-linker-G3) and 9L1H (i.e., DARPin 9.29-linker-G3) [27,28,29], which concurrently interact HER2 subdomains I and IV through a brief glycine-serine linker (G₄S), have demonstrated potent HER2 signaling inhibition [28]. Whereas these constructs supply a robust structural foundation for twin epitope engagement [28, 29], on this research we chosen the monospecific anti-HER2 area IV DARPin G3 for fusion with the anti-VEGF DARPin Abicipar pegol as a proof-of-concept. Monospecific anti-HER2 and anti-VEGF DARPins have been additionally equally cloned into pET28a(+) vectors for comparative research (Determine S1 a–f). The bispecific and monospecific DARPins have been efficiently reworked into E. coli BL21(DE3) cells (Determine S2, Steps 1–2) and recombinant expression was induced utilizing isopropyl β-D-1-thiogalactopyranoside (IPTG). Soluble expression was achieved underneath customary circumstances, with nearly all of the DARPin proteins recovered within the supernatant following bacterial lysis and centrifugation (Determine S2, Steps 3–5). Yields of the bispecific DARPin have been extremely reproducible throughout unbiased batches, averaging roughly 100–130 mg/L (Desk S1).
DARPin purification was carried out utilizing nickel–nitrilotriacetic acid (Ni-NTA) affinity chromatography, exploiting the designed N-terminal 6xHis-tag, adopted by size-exclusion chromatography (SEC) to realize excessive monomeric purity (Determine S2, Steps 6–7). SEC profiles confirmed a predominant monomeric peak for the HER2×VEGF bispecific DARPin conjugate at roughly 30.1 kDa, with monospecific DARPins eluting as anticipated at ~ 15.9 kDa (Fig. 1c). Purified proteins have been additional characterised by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), confirming anticipated molecular weights and the profitable elimination of main contaminants (Fig. 1d). As anticipated, minor dimer formation was detected amongst all three kinds of DARPins expressed, in keeping with intermolecular disulfide bond formation. Beneath non-reducing circumstances, the HER2×VEGF DARPin exhibited 1.7% dimer content material after SEC purification, which was additional diminished to 0.2% and 0 following therapy with 1 mM tris(2-carboxyethyl)phosphine (TCEP) or β-mercaptoethanol (BME), respectively (Desk S2, Fig. 1d). Monospecific anti-HER2 and anti-VEGF DARPins displayed barely greater preliminary dimerization (7.0% and seven.6%, respectively), which was equally reversible upon lowering agent therapy (Desk S2). To guage scalability, we carried out a 10-fold scale-up from a 30 mL tradition (1× scale) to a 300 mL tradition (10× scale) (Determine S3a). SDS-PAGE evaluation underneath non-reducing and lowering circumstances confirmed that the scale-up didn’t adversely have an effect on protein purity or integrity (Determine S3b). Densitometry evaluation revealed comparable monomer content material and minimal dimer formation (1.9% vs. 1.6%), supporting the robustness of the expression and purification processes at bigger scales.
It’s price noting that to facilitate optionally available tag elimination, we engineered our assemble with an N-terminal 6×His-tag adopted by a TEV protease cleavage web site (Fig. 1b, black arrowhead). This design permits for environment friendly and scarless elimination of the His-tag when obligatory. Whereas the His-tag was retained for purposeful research offered herein, together with cell binding assays utilizing circulate cytometry and enzyme-linked immunosorbent assay (ELISA), cleavage protocols and purification methods supporting workflows for tag-free proteins are additionally detailed (see Strategies part). Particularly, post-cleavage purification may be effectively achieved utilizing the same Ni-NTA affinity chromatography, leveraging the His-tag on TEV protease to separate it and the cleaved tag from the goal protein. Moreover, to make sure suitability for downstream organic functions, all DARPin samples underwent customary endotoxin elimination utilizing high-capacity spin columns, and have been confirmed to have endotoxin ranges under the assay’s detection restrict of 0.5 endotoxin models (EU)/mL, as detailed within the Strategies part.
Cloning, expression, and purification of bispecific HER2×VEGF DARPin bioconjugates. (a) Schematic map of the pET28a(+) expression vector containing the HER2×VEGF bispecific DARPin gene (6079 bp) underneath the T7 promoter, with an N-terminal 6×His-tag and TEV protease cleavage web site for affinity purification, and a C-terminal (G3S)2 linker adopted by a cysteine residue for site-specific conjugation. (b) Annotated amino acid sequence of the expressed HER2×VEGF bispecific DARPin, exhibiting the N-terminal 6×His-tag and TEV cleavage sequence (pink), anti-HER2 DARPin area (grey), versatile (G₄S)₃ linker (inexperienced), anti-VEGF DARPin area (orange), and C-terminal cysteine residue (blue). The TEV cleavage web site is marked by a black arrowhead. (c) SEC profiles of the purified HER2×VEGF bispecific DARPin (inexperienced) and monospecific anti-HER2 (grey) and anti-VEGF (orange) DARPins, demonstrating a predominant monomeric species, in contrast in opposition to protein molecular weight requirements (blue). (d) SDS-PAGE evaluation of purified DARPins exhibiting anticipated molecular weights with ~ 30.1 kDa for bispecific HER2×VEGF DARPin, ~ 15.9 kDa for monospecific anti-HER2 and anti-VEGF DARPins), and analysis earlier than and after lowering therapies with 1 mM TCEP or BME
To additional assess the biochemical stability of the HER2×VEGF bispecific DARPin-cysteine conjugate, we carried out sequential SEC evaluation over a 21-day time-course at each 4 °C and 25 °C (Determine S4, Desk S3). Storage at 4 °C maintained a predominant monomeric inhabitants, with monomer content material lowering barely from ~ 100% on Day 0 to 83.9% on Day 21, accompanied by a modest improve in dimer formation from practically zero to 16.1%. In distinction, samples saved at 25 °C exhibited accelerated dimerization, with monomer content material lowering to 59.5% and dimer growing to 40.5% by Day 21 (Desk S3). Importantly, therapy with 1 mM TCEP at Day 21 successfully restored the DARPin to > 99% monomeric species underneath each storage circumstances, confirming that the noticed dimerization was mediated by reversible disulfide bond formation. To additional validate these findings, we analyzed the identical samples by non-reducing and lowering SDS-PAGE (Determine S5). Densitometry evaluation confirmed that dimer content material at Day 21 was 20.8% for samples saved at 4 °C and 37.6% for samples saved at 25 °C, carefully matching the SEC quantification (Desk S3). Beneath lowering circumstances, all samples reverted predominantly to monomeric kinds, additional supporting the disulfide-mediated mechanism of dimerization.
To enrich these stability assessments, thermal denaturation of the HER2×VEGF bispecific DARPin-cysteine conjugate was evaluated utilizing round dichroism (CD) spectroscopy at 222 nm, a wavelength delicate to α-helical content material [26]. The unfolding curve displayed a pointy and cooperative transition with a melting temperature (Tm) of roughly 74.5 °C (Determine S6), in keeping with a two-state folding mannequin [26, 30]. A slight gradual decline in ellipticity was noticed from 20 °C to ~ 70 °C, reflecting refined pre-transition structural fluctuations, as reported for different thermostable ankyrin repeat proteins [31]. These information verify the excessive thermal stability and conformational integrity of the DARPin scaffold, supporting its robustness for site-specific conjugation and superior biomedical functions.
These outcomes collectively reveal that the HER2×VEGF bispecific DARPin-cysteine conjugate possesses good storage stability, notably underneath refrigerated circumstances, and that any dimerization is basically reversible by delicate discount. General, the HER2×VEGF bispecific DARPin demonstrated glorious recombinant expression effectivity, excessive purity after dual-step purification, and robust biochemical stability, offering a strong platform for subsequent bioconjugation and purposeful analysis.
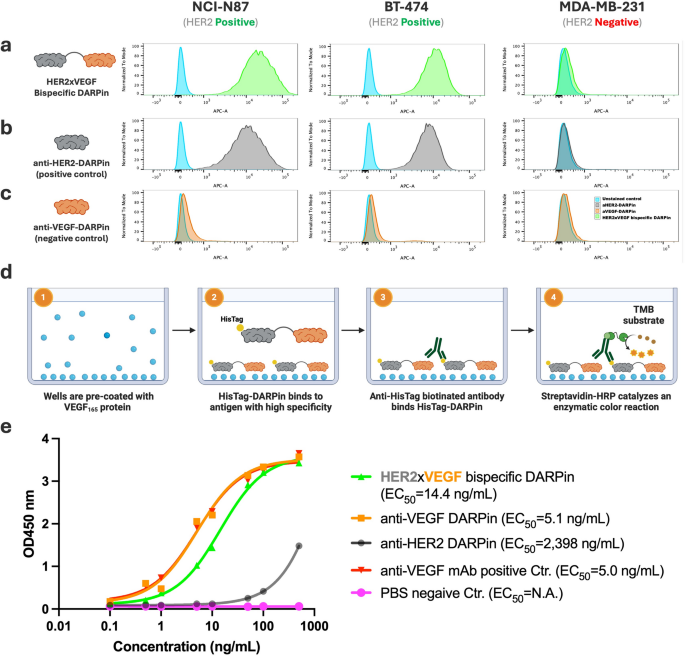
Practical analysis of HER2×VEGF bispecific and monospecific DARPin binding actions by circulate cytometry and ELISA. (a–c) Move cytometry evaluation of DARPin binding to HER2-positive (NCI-N87, BT-474) and HER2-negative (MDA-MB-231) breast most cancers cell strains. Cells have been incubated with (a) HER2×VEGF bispecific DARPin, (b) anti-HER2 monospecific DARPin (optimistic management), or (c) anti-VEGF monospecific DARPin (damaging management). Binding was detected through anti-HisTag APC-conjugated antibody. (d) Schematic illustration of the anti-VEGF ELISA assay setup. (1) Wells are pre-coated with recombinant human VEGF165 protein. (2) His-tagged DARPins (HER2×VEGF bispecific or monospecific controls) bind particularly to the immobilized VEGF165 antigen. (3) Biotinylated anti-HisTag antibody acknowledges the certain DARPins, adopted by (4) streptavidin-HRP binding and catalysis of a colorimetric response utilizing TMB substrate for detection. (e) ELISA outcomes exhibiting concentration-dependent binding of HER2×VEGF bispecific DARPin and anti-VEGF monospecific DARPin to VEGF165, however no detectable binding by the anti-HER2 monospecific DARPin. Industrial anti-VEGF mAb-biotin and phosphate-buffered saline (PBS) have been chosen to function optimistic and damaging controls for assay validation. EC50 values have been decided by nonlinear regression evaluation
HER2/VEGF focused binding assay of DARPins
To substantiate the retention of dual-targeting functionality within the designed HER2×VEGF bispecific DARPin, we evaluated binding to each HER2 and VEGF utilizing established cell-based and ELISA assays. Monospecific anti-HER2 and anti-VEGF DARPins have been used as optimistic controls, together with commercially accessible anti-HER2 and anti-VEGF monoclonal antibodies. First, we chosen human NCI-N87 gastric and BT-474 breast most cancers cell strains, that are broadly used HER2-positive fashions, and MDA-MB-231 as a HER2-negative human triple damaging breast most cancers management line. The HER2 expression standing of those cell strains was validated by circulate cytometry utilizing a commercially accessible anti-HER2 PE-conjugated monoclonal antibody (PE-mAb). As anticipated, NCI-N87 and BT-474 exhibited robust HER2-specific staining, whereas MDA-MB-231 confirmed minimal HER2 expression (Determine S7a–c).
We then assessed HER2-binding exercise of the DARPin constructs by circulate cytometry utilizing an APC-conjugated anti-HisTag monoclonal antibody to detect DARPin binding. Cells have been incubated with 10 nM of both the bispecific DARPin or the respective monospecific DARPins (anti-HER2 or anti-VEGF) at 4 °C for 30 min to permit binding. The HER2×VEGF bispecific DARPin confirmed particular binding to HER2-positive NCI-N87 and BT-474 cells, with no detectable binding to HER2-negative MDA-MB-231 cells (Fig. 2a). Equally, the anti-HER2 monospecific DARPin confirmed selective binding to HER2-positive cells (Fig. 2b), whereas the anti-VEGF monospecific DARPin exhibited no binding to any of the examined cell strains (Fig. 2c), in keeping with the absence of VEGF floor expression on these cell strains.
In parallel, we developed a VEGF-specific ELISA assay to evaluate VEGF-binding functionality. Wells have been pre-coated with recombinant human VEGF165 protein, and DARPin binding was detected utilizing biotinylated anti-HisTag antibody, adopted by streptavidin-horseradish peroxidase (HRP) and three,3′,5,5′-tetramethylbenzidine (TMB) colorimetric detection (Fig. 2d). The HER2×VEGF bispecific DARPin demonstrated concentration-dependent binding to VEGF165 (EC50 = 14.4 ng/mL), corresponding to the anti-VEGF monospecific DARPin (EC50 = 5.1 ng/mL), whereas the anti-HER2 monospecific DARPin confirmed no clear VEGF binding (EC50 > 2000 ng/mL) (Fig. 2e). Industrial anti-VEGF mAb-biotin and PBS served as optimistic and damaging controls, respectively, validating the assay specificity. Collectively, these outcomes verify that the engineered HER2×VEGF bispecific DARPin retains particular binding to each HER2 and VEGF targets, with comparable efficiency to monospecific DARPin controls.
Website-specific azide modification of HER2×VEGF bispecific DARPins
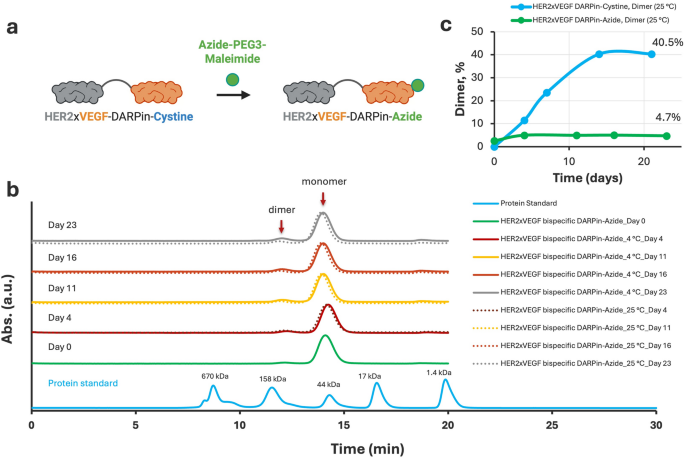
To allow downstream site-specific conjugation to nanodelivery automobiles and enhance biochemical stability, we selectively modified the free thiol group of the C-terminal cysteine on the HER2×VEGF bispecific DARPin utilizing an Azide-PEG3-Maleimide linker. The thiol–maleimide response effectively launched an azide performance to the DARPin, producing the HER2×VEGF bispecific DARPin-azide assemble (Fig. 3a). Following conjugation, SEC purification was carried out to isolate the monomeric DARPin-azide product and take away unreacted linkers or minor byproducts. The ultimate HER2×VEGF bispecific DARPin-azide conjugate achieved a monomeric purity higher than 98% as assessed by SEC-high-performance liquid chromatography (HPLC) evaluation (Determine S8), indicating profitable modification and glorious product homogeneity.
Website-specific modification of HER2×VEGF bispecific DARPin-Cysteine with Azide-PEG₃-Maleimide and comparability of stability profiles earlier than and after conjugation. (a) Schematic illustration of the conjugation technique: the free thiol group of the C-terminal cysteine within the HER2×VEGF bispecific DARPin-cysteine conjugate was selectively modified with azide-PEG3-maleimide by means of a thiol–maleimide coupling response, producing a HER2×VEGF bispecific DARPin-azide conjugate. (b) SEC profiles monitoring the soundness of HER2×VEGF bispecific DARPin-azide conjugates over 23 days at 4 °C (strong strains) and 25 °C (dotted strains). (c) Quantitative comparability of dimer content material throughout storage at 25 °C, highlighting the substantial suppression of dimerization following Azide-PEG3 modification, in distinction to the progressive dimer accumulation seen with the unmodified DARPin-cysteine assemble
The soundness of the HER2×VEGF bispecific DARPin-azide assemble was then evaluated over a 23-day interval at each 4 °C and 25 °C (Fig. 3b). In distinction to the guardian HER2×VEGF bispecific DARPin-cysteine conjugate, which confirmed progressive dimerization throughout storage (Determine S4), the azide-modified DARPin conjugate demonstrated exceptional biochemical stability, with minimal dimer formation, even underneath elevated temperature circumstances. Quantitative evaluation of dimer content material at 25 °C revealed a hanging distinction: whereas the unmodified DARPin-cysteine gathered as much as 40.5% dimer content material by Day 21 (Determine S4), the DARPin-azide conjugate maintained lower than 5% dimer even after 23 days at 25 °C (Fig. 3c). This important suppression of dimerization is attributed to the profitable blocking of the reactive C-terminal cysteine through thiol–maleimide chemistry, stopping disulfide-mediated intermolecular crosslinking. General, these outcomes verify that site-specific azide-PEG3-maleimide modification not solely allows orthogonal click on chemistry conjugation for future biomedical and translational functions, however dramatically enhances the biochemical stability and homogeneity of the HER2×VEGF bispecific DARPin underneath storage circumstances.
Proof-of-Idea bioconjugation of HER2×VEGF bispecific DARPin for nanobiotechnology functions
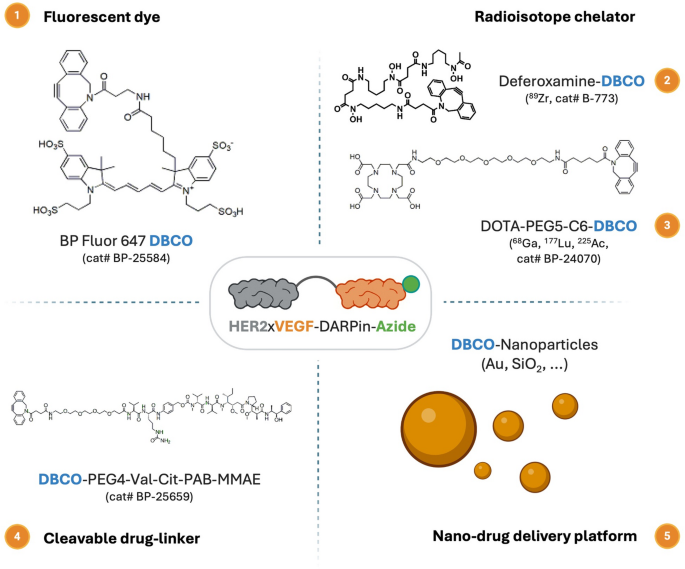
To develop the utility of the engineered HER2×VEGF bispecific DARPin, we demonstrated its compatibility with orthogonal click on chemistry-mediated conjugations. Particularly, the azide-functionalized HER2×VEGF bispecific DARPin (HER2×VEGF DARPin-azide) enabled environment friendly strain-promoted azide–alkyne cycloaddition (SPAAC) reactions with varied dibenzocyclooctyne (DBCO)-functionalized moieties, together with fluorescent dyes, radiometal chelators, drug-linkers, and nanoparticle platforms (Scheme 1, Desk S4). This modular conjugation method provides a flexible platform for biomedical functions, together with, however not restricted to, optical imaging, positron emission tomography (PET) imaging, focused radionuclide remedy, and superior nanodrug supply methods.
Schematic overview of potential functions for HER2×VEGF bispecific DARPin-azide bioconjugates by means of orthogonal click on chemistry bioconjugation. The azide-functionalized HER2×VEGF bispecific DARPin bioconjugate can endure SPAAC click on chemistry reactions with numerous DBCO-modified payloads, together with (1) fluorescent dyes for optical imaging (e.g., BP Fluor 647-DBCO), (2) radioisotope chelators for PET/SPECT (single photon emission computed tomography) imaging or radiotherapy (e.g., deferoxamine-DBCO and DOTA-PEG5-C6-DBCO), (3) cleavable cytotoxic drug linkers (e.g., DBCO-PEG4-Val-Cit-PAB-MMAE) for focused remedy, and (4) DBCO-functionalized nanoparticles (e.g., Au, SiO2 nanoparticles) for superior nanomedicine supply platforms
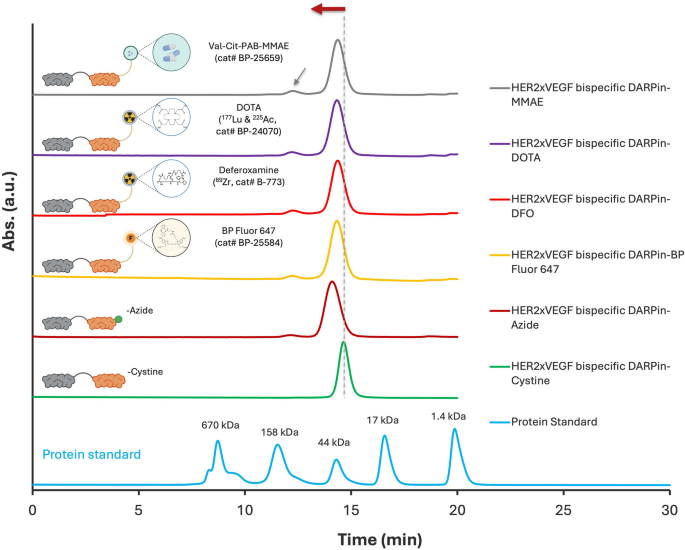
The profitable site-specific conjugation of HER2×VEGF bispecific DARPin-azide to numerous DBCO-functionalized moieties—together with fluorophores, chelators, and a cleavable MMAE drug linker—was validated by each SEC-HPLC and SDS-PAGE analyses. SEC-HPLC profiles (Fig. 4) demonstrated distinct left-shifted elution peaks for all conjugated DARPins in comparison with the unmodified DARPin-azide and DARPin-cysteine controls, indicating elevated obvious molecular weight in keeping with profitable covalent coupling. All conjugates present dominant monomeric purity. A minor dimer peak (grey arrowhead) constantly noticed throughout all profiles possible originates from a small fraction (< 2%) of unconjugated HER2×VEGF DARPin-cysteine (Determine S8), which was retained in all bioconjugated samples. Complementary SDS-PAGE evaluation underneath non-reducing and lowering circumstances (Determine S9) additional supported the technology of well-defined, high-purity conjugates. Every functionalized variant displayed a clear, anticipated banding sample with no proof of degradation or side-product formation, underscoring the robustness and orthogonality of the press chemistry technique. Determine S10 additional demonstrates environment friendly and particular bioconjugation of BP Fluor 647 to the HER2×VEGF bispecific DARPin, as confirmed by co-elution of absorbance peaks at each 280 nm and 651 nm throughout SEC-HPLC dual-wavelength evaluation. These information collectively reveal that the C-terminal azide-engineered HER2×VEGF bispecific DARPin serves as a flexible scaffold for producing homogeneous, high-purity conjugates appropriate for numerous biomedical functions.
SPR assay improvement and validation of monospecific DARPins
To precisely characterize the bispecific binding conduct of HER2×VEGF DARPins and assess the influence of site-specific bioconjugation, we first established and validated a floor plasmon resonance (SPR) assay utilizing monospecific DARPin controls. This step ensured that the immobilized HER2 and VEGF165 antigens have been functionally energetic and selectively acknowledged by their respective DARPins. Recombinant human HER2 or VEGF165 proteins have been immobilized independently on carboxymethylated dextran sensor chip (CM5) through customary amine coupling, and analytes have been injected at concentrations starting from 0.16 to 160 nM. The binding information have been analyzed utilizing a 1:1 Langmuir binding mannequin to extract kinetic parameters (okayon, okayoff) and equilibrium dissociation constants (OkD).
SEC-HPLC evaluation confirming profitable conjugation of HER2×VEGF bispecific DARPin-Azide to totally different DBCO-functionalized moieties. SEC-HPLC chromatograms demonstrating environment friendly bioconjugation to (high to backside): cleavable MMAE drug linker, DOTA chelator, deferoxamine chelator, BP Fluor 647 dye, in comparison with the unmodified DARPin-Azide and DARPin-Cysteine precursors. All conjugates present dominant monomeric purity. A minor dimer peak (grey arrowhead) possible derived from residual unconjugated DARPin-Cysteine was constantly current throughout all bioconjugated samples
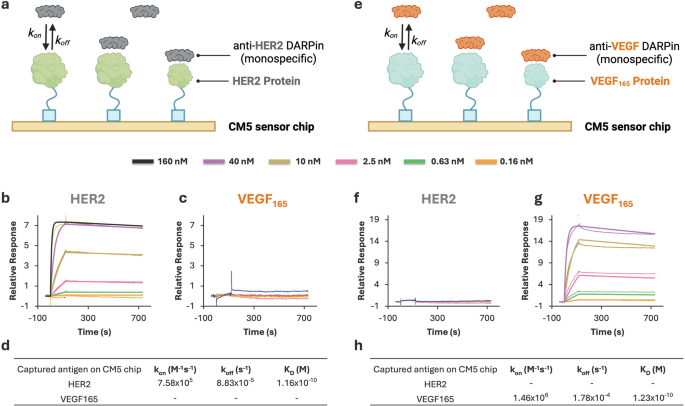
SPR assay improvement and validation for monospecific DARPins. (a) Schematic of SPR setup with HER2 antigen immobilized on a CM5 chip and anti-HER2 DARPin because the analyte. (b–c) Sensorgrams exhibiting binding of anti-HER2 DARPin to HER2-immobilized chip (b) and no detectable interplay with VEGF165-immobilized chip (c). (d) Kinetic parameters (okayon, okayoff, and OkD) for anti-HER2 DARPin interplay with HER2. (e) Schematic of SPR setup with VEGF165 antigen immobilized on a CM5 chip and anti-VEGF DARPin because the analyte. (f–g) Sensorgrams exhibiting no detectable binding of anti-VEGF DARPin to HER2-immobilized chip (f) and particular interplay with VEGF165-immobilized chip (g). (h) Kinetic parameters (okayon, okayoff, and OkD) for anti-VEGF DARPin interplay with VEGF165
As proven in Fig. 5, the anti-HER2 monospecific DARPin assemble exhibited robust, particular binding to the HER2-coated floor (OkD= 1.16 × 10−10 M, or 116 pM), with no detectable interplay on VEGF165-coated chips. Conversely, the anti-VEGF DARPin assemble certain completely to VEGF165 (OkD = 1.23 × 10−10 M, or 123 pM) and confirmed no response on HER2-immobilized surfaces. These outcomes verify the specificity of the DARPins and validate that every chip floor was appropriately ready and functionally selective.
This assay validation offers a strong basis for subsequent kinetic analyses of HER2×VEGF bispecific DARPin conjugates. By immobilizing HER2 and VEGF165 antigens individually, we will resolve and quantify binding to every goal independently. This platform allows exact evaluation of bispecific binding conduct in each unmodified and bioconjugated DARPins and helps direct comparisons of binding affinity and kinetics throughout quite a lot of purposeful modifications. In all subsequent research, kinetic constants have been extracted utilizing the validated 1:1 Langmuir mannequin, making certain constant and reproducible evaluation of binding interactions.
Twin antigen concentrating on by purposeful HER2×VEGF bispecific DARPin bioconjugates
To guage the dual-targeting functionality of HER2×VEGF bispecific DARPin conjugates engineered with modular click-reactive websites, we carried out SPR assays utilizing the validated CM5 sensor chips with independently immobilized HER2 or VEGF165 antigens (Fig. 6a). Following the assay improvement technique established earlier, we characterised binding responses of a number of conjugated DARPin variants functionalized for molecular imaging or therapeutic use, together with fluorophores, radiometal chelators, and cytotoxic drug-linkers (Fig. 6b–m). Consultant kinetic constants are summarized in Desk 1.
We first confirmed the high-affinity binding of the unmodified HER2×VEGF DARPin-cysteine conjugate and its site-specifically modified spinoff, a DARPin-azide conjugate. Each constructs demonstrated dual-target engagement with picomolar-to-subnanomolar equilibrium dissociation constants: OkD= 77.4 pM for HER2 and 338 pM for VEGF165 for the DARPin-cysteine assemble. These values signify potent antigen engagement, in step with reported high-affinity DARPins in opposition to HER2 and VEGF [23, 25]. Importantly, the binding affinities of each DARPin constructs in contrast favorably with these of commercially accessible biotinylated monoclonal antibodies, specifically anti-HER2 antibody (cat# BAF1129, OkD = 524 pM) and anti-VEGF antibody (cat# BAF293, OkD = 52 pM) (Desk 1). Notably, sensorgrams for these two constructs displayed extraordinarily gradual dissociation phases (Determine S11 c–g), a recognized limitation in SPR evaluation of very robust binders comparable to DARPins [32]. These observations reinforce the robustness of the paratope structure, even after modular cysteine-azide substitution.
Subsequent SPR analyses of functionally modified HER2×VEGF bispecific DARPins—together with conjugates bearing BP Fluor 647 dye, DOTA (1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid), deferoxamine (DFO), and a cleavable MMAE linker—demonstrated preserved binding to each HER2 and VEGF165, with equilibrium dissociation constants (OkD) starting from roughly 1 to three nM (Desk 1). In comparison with the guardian DARPin-cysteine assemble, the conjugates retained comparable affiliation charges (okayon), whereas exhibiting modestly quicker dissociation charges (okayoff), accounting for the slight discount in total affinity. Regardless of this shift, all constructs maintained clinically related low-nanomolar binding capability for each targets. These outcomes point out that site-specific click-based functionalization on the engineered distal cysteine web site imposes minimal structural or steric disruption to the antigen-binding interface. This kinetic development is in keeping with prior research of engineered DARPins, comparable to PEGylated and dye-labeled variants concentrating on epithelial cell adhesion molecule (EpCAM) [33], which additionally confirmed preserved specificity and solely minor modifications in purposeful affinity following bioconjugation. Collectively, these findings assist the robustness of our HER2×VEGF bispecific DARPin scaffold and validate its modular structure as a flexible platform for focused imaging and therapeutic functions.