Preparation and characterization of Ag2S tremendous NPs
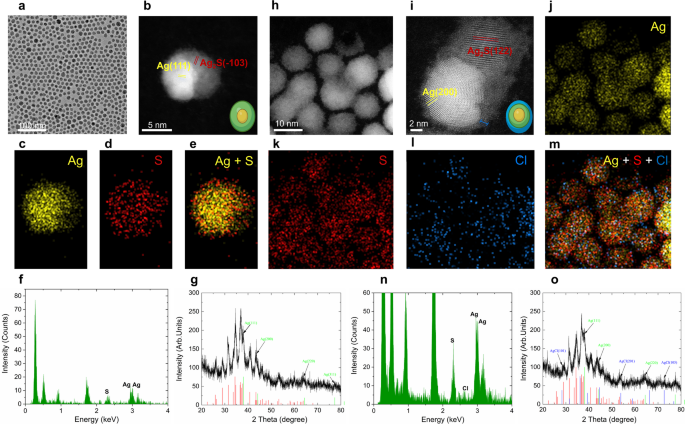
Microwaves, a type of nonionizing electromagnetic vitality, reveals a spectral frequency vary of 300–300,000MHz [41]. Microwave irradiation is able to accelerating chemical reactions which can be derived from its excessive vitality [42]. On this work, we employed a power-tunable microwave oven to supply the required microwave vitality. We fabricated Ag2S tremendous NPs by irradiating a chemically synthesized Ag/Ag2S heterodimers (hereafter known as Ag2S NPs) dispersed in CHCl3 with microwave. It’s value noticing that aspect merchandise, Ag NPs (DetermineS1), are generated by way of our chemical synthesis methodology (Experimental Part). This phenomenon is attributed to the excessive redox potential of Ag+ on the synthesis temperature [43]. These Ag NPs in CHCl3 could be remodeled into AgCl below the irradiation of microwave. Determine 2 illustrated the morphology and elemental evaluation of Ag2S NPs earlier than and after irradiation of microwave. Determine 2a depicts the picture of transmission electron microscope (TEM) of Ag2S NPs earlier than irradiation. All of the NPs exhibit an elliptical morphology with a mean dimension of roughly 10 nm. One may discover that two distinct contrasts exist inside particular person NPs, suggesting the formation of two completely different phases inside a single NP. To find out the precise composition of the Ag2S NPs, a high-resolution scanning electron microscope (HRSTEM, Fig. 2b) and elemental evaluation (Fig. 2c-e) had been carried out. The HRTEM picture of a single Ag2S NP reveals an identical yolk-shell crystalline construction, characterised by an electron-dense core and an electron-transparent shell (Fig. 2b). The lattice fringe spacings are d (111) = 2.34 Å for the core half and d (−103) = 2.38 Å for the outer shell. These values correspond nicely to these of cubic Ag (JCPDS No. 04-0783) and monoclinic Ag2S (JCPDS No. 14–0072) respectively, in flip, directing that the internal core consists of Ag whereas the outer shell consists of Ag2S. The sunshine and darkish contrasts within the HRTEM picture come up from the decrease density of Ag2S (7.2 g/cm3) in comparison with Ag (10.50 g/cm3). The basic mapping (Fig. 2c-e) demonstrated that core is wealthy in Ag whereas S is predominantly distributed within the outer shell a part of the synthesized Ag2S NPs. This conclusion is additional supported by energy-dispersive X-ray spectroscopy (EDX) evaluation (Fig. 2f), presenting a Ag: S ratio of 64:36 for a Ag2S NP. Furthermore, X-ray diffraction (XRD) sample in Fig. 2g confirms each cubic-phase Ag and monoclinic-phase Ag2S, supporting the aforementioned elemental and structural evaluation. Collectively, these outcomes point out that the existence of a Ag core throughout the as-synthesized Ag2S NPs.
After the irradiation of microwave at 210 W for two min, the typical diameter of the NPs elevated from 10 nm to 12 nm (Fig. 2h). The HRTEM picture in Fig. 2i reveals two forms of lattice fringe: a dense core with a lattice spacing of d (200) = 2.04 Å directing to cubic Ag and a lighter shell with a lattice distance of d (122) = 2.09 Å akin to monoclinic Ag2S. Notably, an amorphous layer roughly 1 nm thick surrounds the NPs, as noticed in HRSTEM picture. Elemental mapping in Fig. 2(j-l) reveals that the irradiated nanoparticles include Ag and S, just like the Ag2S NPs, with Cl moreover distributed all through the microwave-irradiated Ag2S NPs (Fig. 2m). That is attributed to the formation of a AgCl layer. The presence of AgCl is additional evidenced by the EDX evaluation (Fig. 2n) and XRD sample (Fig. 2o). The microwave-irradiated Ag2S NPs exhibit a Ag: S:Cl ratio of 63:31:6. These findings reveal that the Ag2S NPs are composed of cubic Ag and monoclinic Ag2S. Upon microwave irradiation, their composition and construction modified. The distinct formation of a brand new amorphous AgCl skinny outer layer as noticed in Fig. 2i and o.
The morphology and elemental evaluation of Ag2S NPs earlier than and after irradiation of microwave. (a) TEM picture of Ag2S NPs by way of chemical synthesis course of, (b) high-resolution STEM picture of a single Ag2S NP presenting lattice fringes d111 and d−103 direct to cubic Ag and monoclinic Ag2S, respectively, elemental mapping of the distribution of Ag (c), S (d) and Ag + S (e) of a Ag2S NP, (f) EDX spectrum of Ag2S NPs earlier than microwave irradiation and corresponding XRD sample (g), (h) HADDF-STEM picture of Ag2S NPs after microwave irradiation at 210 W for two min, (i) high-resolution STEM picture of a microwave-irradiation Ag2S NP with lattice fringes d200 and d122 stem to cubic Ag and monoclinic Ag2S, respectively, the amorphous shell across the NP was marked with blue line, elemental mapping of the distribution of Ag (j), S (ok), Cl (l) and Ag + S + Cl (m) of a microwave-irradiation Ag2S NPs, corresponding EDX spectrum (n) and XRD sample (o)
Optical properties of Ag2S tremendous NPs and enhancement mechanism
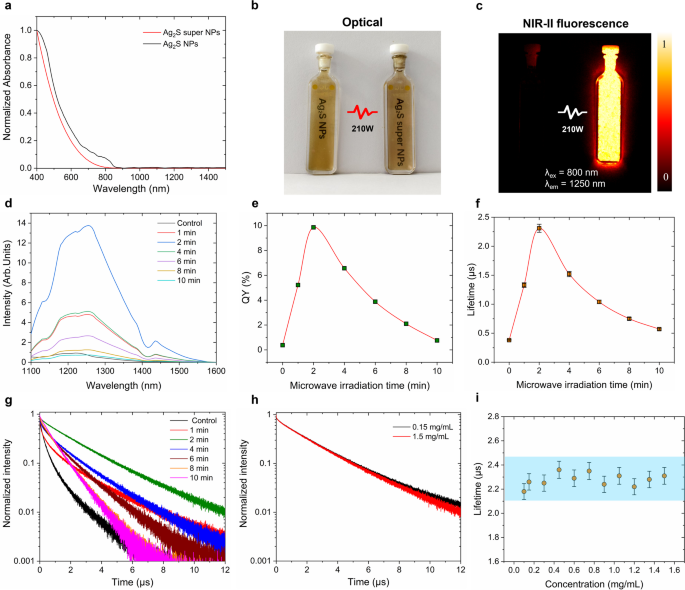
After the irradiation of microwave, the structural modifications, illustrated in Fig. 2, considerably improve their optical properties. Microwave irradiation reduces the absorbance of the samples on the vary of 400–800 nm to 30% as proven in Fig. 3a, and this discount is carefully correlated with the irradiation time below 210 W (Determine S2). The absorption spectrum of Ag2S NPs earlier than remedy reveals a attribute shoulder round 400 nm which could be attributed to the plasmonic band of Ag2S NPs at a smaller scale [44]. This characteristic disappears after the microwave irradiation. As depicted in Fig. 3b, the optical photos of Ag2S NPs earlier than and after microwave irradiation (210 W, 2 min) dispersed in CHCl3 at 1.5 mg/mL reveal that there isn’t a distinct shade change. The brightness of Ag2S NPs, nonetheless, is remarkably promoted by microwave irradiation as evidenced by the NIR-II fluorescence picture (Fig. 3c). In distinction, it’s difficult to detect the NIR-II fluorescence sign to chemically synthesized Ag2S NPs until the imaging of the irradiated pattern is deliberately overexposed. Determine 3d presents the spectra of Ag2S NPs irradiated at 210 W for various durations (1, 2, 4, 6, 8, and 10 min). One may discover that the emission depth of the irradiated Ag2S NPs will increase in comparison with untreated ones, reaching its most enhancement when handled with microwave for two min, adopted by a gradual decrement with extended irradiation time. The spectral form stays constant earlier than and after irradiation, suggesting that the chemical composition is unchanged below microwave irradiation. Nonetheless, the height place presents a purple shift (from 1220 nm to 1255 nm, Determine S3). This tiny purple shift phenomenon might be owing to the dissociation of CHCl3 forming reactive chloride, resulting in excessive chloride protection on the floor of Ag2S NPs [45, 46]. Furthermore, the enhancement in emission depth is corroborated by the fluorescence quantum yield (QY, Fig. 3e) and fluorescence lifetime (Fig. 3f) outcomes obtained from various durations of microwave irradiation. The QY begins to extend upon publicity to 1-min microwave irradiation and reaches its most when uncovered to 2-min irradiation. Subsequently, the QY decreases with additional will increase in irradiation time, a development that carefully aligns with the modifications in fluorescence emission. Determine S4 contains consultant excitation and emission spectra which were utilized for QY calculations. Particularly, the QY of the synthesized Ag2S NPs will increase from 0.38 to 9.8% after 2-min microwave irradiation at 210 W, representing roughly a 25-fold enhancement. This vital promotion facilitates the microwave-induced transformation of Ag2S NPs into Ag2S tremendous NPs. Correspondingly, the fluorescence lifetime extends from 0.37 µs as much as 2.31 µs, as proven in Fig. 3f. The related decay curves are offered in Fig. 3g. Notably, this lifetime enhancement stays constant no matter variations within the focus of Ag2S tremendous NPs, in flip, eliminating the potential self-absorption impact on the lifetime measurements. (Fig. 3h-i).
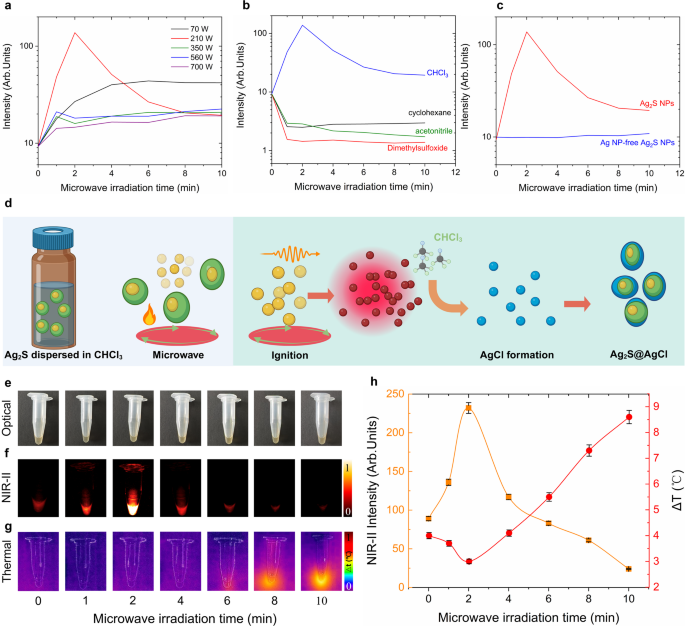
To discover the brightness enhancement mechanism and optimum experimental circumstances of microwave-irradiation-induced Ag2S tremendous NPs, a collection of important experiments had been carried out. Since fluorescence depth is extremely depending on pattern focus, all of the samples had been standardized to 1.5 mg/mL after microwave irradiation. Determine 4a presents the time-evolution fluorescence depth of Ag2S NPs irradiated below numerous microwave energy as much as 10 min, corresponding fluorescence spectra are supplied in Determine S5. One may discover that the NIR-II emission depth will increase with irradiation time when the microwave energy ranges between 70 and 210 W and irradiation time is lower than 4 min. Past this threshold, the fluorescence depth stays practically steady even when the irradiation time is extended. Notably, on the irradiation energy of 210 W, a 14-fold depth enhancement was achieved inside 2 min, adopted by a gradual lower over extended irradiation occasions. This may be attributed to the degradation of the Ag2S NPs and thermal quench impact below extended publicity to 210 W microwave irradiation [28]. When the microwave energy exceeds 210 W, the fluorescence depth could be enhanced fairly restricted for 1-min irradiation. Subsequently, the depth decreases with extended irradiation till it reaches a steady worth. This decrement, on the one hand, primarily owing to the thermal quench impact below high-power microwave, alternatively, could be as a result of uncomplete response attributable to the fast evaporation of the solvent (CHCl3) below high-power microwave irradiation after 1 min. Contemplating these circumstances, the colour of the samples dispersed in CHCl3 wouldn’t have a major distinction. The consultant optical pictures of the untreated and handled (on the energy of 70, 210, 350, 560 and 700 W) samples redispersed in CHCl3 to attain the identical focus of 1.5 mg/mL are offered in Determine S6. These outcomes, in flip, verify that the optimum situation for enhancing fluorescence depth is 210 W irradiation for two min.
The fluorescence property characterization of Ag2S NPs earlier than and after microwave irradiation. (a) Absorption spectra of Ag2S NPs earlier than (black) and after (purple) irradiation of microwave below 210 W for two min, (b)optical photos of Ag2S NPs earlier than (left) and after (proper) irradiation of microwave below 210 W for two min, (c) corresponding NIR-II fluorescence photos in (b), (d) NIR-II fluorescence spectra of Ag2S NPs with the microwave irradiation at 210 W for various occasions, corresponding QY values (e) and fluorescence lifetime values (f) in (d), (g) corresponding fluorescence lifetime decay curves of Ag2S NPs after microwave irradiation at 210 W for various time intervals, (h) fluorescence lifetime curves of Ag2S NPs at completely different focus after microwave irradiation at 210 W for two min, (i) lifetime values of Ag2S NPs after microwave irradiation at 210 W for two min as a operate of focus. All of the measurements had been obtained in a dispersion of CHCl3. The error bars in (i) symbolize three paralleled measurements
For the aim of investigating whether or not the solvent properties would have an effect on the transformation, we chosen a number of generally used natural solvents, together with cyclohexane, dimethylsulfoxide (DMSO) and acetonitrile, for additional analysis. Determine 4b demonstrates that the microwave-induced transformation of Ag2S NPs happens solely when the NPs are dispersed in CHCl3. When the NPs dispersed in cyclohexane, dimethylsulfoxide (DMSO) or acetonitrile, the microwave irradiation ends in a lower of their NIR-II fluorescence, suggesting the first trigger is the interaction between microwave-induced thermal loading and the intrinsic thermal quenching of the NIR-II emission of Ag2S NPs (corresponding fluorescence spectra had been included in Determine S7). Furthermore, the presence of cubic silver core inside Ag2S NPs is essential for the NPs-to-super NPs transformation. As proven in Fig. 4c, microwave irradiation wouldn’t improve the fluorescence depth of the Ag NP-free Ag2S NPs (corresponding fluorescence spectra had been included in Determine S7). Due to this fact, for the microwave-induced NPs-to-super NPs transformation, each the presence of CHCl3 and the existence of Ag NPs are important.
At this stage, summarizing all of the above experimental findings, a doable schematic clarification for the NPs-to-super NPs transformation is offered in Fig. 4d. Upon microwave irradiation, the excessive vitality absorption by Ag NPs [47] triggers their degradation, resulting in a fast improve within the focus of extremely reactive Ag+ ions within the answer. It’s value noticing that the Ag core wouldn’t be affected by microwave irradiation because of its encapsulation by non-microwave-absorbing Ag2S. Subsequently, these Ag+ ions are able to reacting with solvent CHCl3 to kind AgCl, which might be deposited onto the floor of Ag2S NPs below the assistant of microwave irradiation. Because of this, after microwave irradiation, low-bandgap Ag2S (0.9 eV) is protectively coated by the high-bandgap (5.13 eV) inorganic shell AgCl. The AgCl shell, on the one hand, protects the Ag2S NPs by way of the discount of non-radiative transition induced by the solvent CHCl3 quenching impact. Alternatively, it permits stopping the non-radiative deexcitation pathways attributable to potential defects on the floor or internal construction. Furthermore, the numerous discount of non-radiative transition additional diminishes the portion of light-to-heat conversion effectivity of Ag2S tremendous NPs (Fig. 4e-h), which is additional supported by the light-to-heat conversion comparability experiment with low-bright business Ag2S NPs (Determine S8).
Enhancement mechanism exploration of the Ag2S NPs after irradiation with microwave. (a) NIR-II emitting fluorescence depth of Ag2S NPs irradiated with microwave at completely different energy (70, 210, 350, 560 and 700 W) for various time (0, 1, 2, 4, 6, 8, 10 min), (b) NIR-II fluorescence depth of Ag2S NPs dispersed in numerous solvent irradiated with 210 W microwave for two min, (c) NIR-II fluorescence depth of Ag2S NPs with and with out Ag core irradiated with 210 W microwave for two min, (d) schematic illustration of the enhancement mechanism of Ag2S tremendous NPs, (e) Optical photos of Ag2S NPs dispersed in CHCl3 earlier than and after irradiation with microwave at 210 W for various time (1, 2, 4, 6, 8, 10 min), corresponding NIR-II photos (f) and thermal photos (g), (h) quantification of NIR-II emitting depth in (f) and temperature increment in (g) as a operate of microwave irradiation time. Error bars in (h) are given by the thermal decision of the thermal digital camera and by the depth fluctuations of the infrared digital camera used for the acquiring of NIR-II fluorescence photos
In vivo NIR-II imaging efficiency of Ag2S tremendous NPs
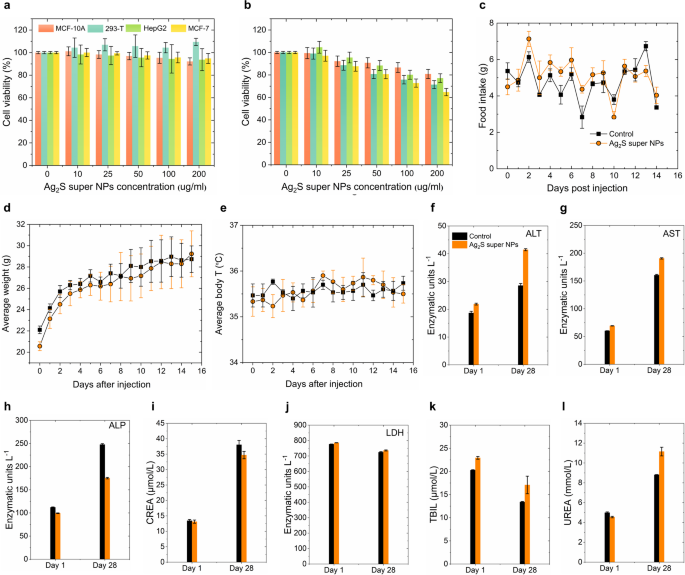
For the following in vivo efficiency analysis, reaching an ideally hydrophilic floor modification of Ag2S tremendous NPs is the precedence. Herein, we employed a ligand trade methodology (Experimental Part in Supporting Info) to switch HS-PEG-COOH onto the floor of Ag2S tremendous NPs, enabling them to switch into the phosphate buffer saline (PBS) for additional investigation. The hydrodynamic dimension distribution of PEG-Ag2S tremendous NPs dispersed in numerous media (water, PBS, serum, DMEM with serum and DMEM) was characterised by dynamic mild scattering (DLS, Determine S9). The DLS outcomes reveal that the typical hydrodynamic dimension of PEG-Ag2S tremendous NPs in numerous media is round 200 nm and reveals stability over a 5-hour steady monitoring interval. One may discover that the DLS ends in Determine S9 exhibit a fluctuation round 100 nm in serum and DMEM, which could be attributed to the distinct aggregation behaviors induced by the various biomolecular and ionic environments current in these media. By way of quantitative evaluation utilizing inductively coupled plasma mass spectrometry (ICP-MS), we investigated the Ag⁺ launch kinetics of PEG-Ag2S tremendous NPs below simulated physiological circumstances at pH 7.4 and weakly acidic circumstances at pH 5.5 (Determine S10). The experimental outcomes revealed no vital Ag⁺ launch below both pH situation, suggesting that the PEG-Ag2S tremendous NPs possesses wonderful stability in ion launch habits. These findings, in flip, reveal that PEG-Ag2S tremendous NPs exhibit favorable biostability and maintain promise for in vivo functions. Moreover, the cytotoxicity of the PEG-Ag2S tremendous NPs was accessed throughout completely different cell traces (MCF-10 A, 293-T, HepG-2 and MCF-7), as proven in Fig. 5a-b. Completely different cells had been incubated with PEG-Ag2S tremendous NPs at concentrations of 10, 25, 50, 100, and 200 µg/mL for twenty-four h (Fig. 5a) and 48 h (Fig. 5b) respectively. The MTT assay outcomes revealed that the PEG-Ag2S tremendous NPs exhibit no vital cell toxicity at excessive concentrations as much as 200 µg/mL inside 48 h, making the PEG-Ag2S tremendous NPs a great candidate for in vivo functions. Furthermore, the in vivo biocompatibility of PEG-Ag2S tremendous NPs was systematically evaluated. Particularly, we monitored the time-evolution in physique weight, meals consumption and physique temperature of the three 4-week Kunming mice for 15 days after intraperitoneal injection of PEG-Ag2S tremendous NPs dispersed in PBS. The entire injection dose for every mouse is 150 µg (300 µL, 0.5 mg/mL, ~ 6 mg/kg), and three extra mice had been injected with 300 µL PBS as a management group. As illustrated in Fig. 5c-e, regardless of a comparatively excessive injection dose, no vital variations had been noticed between the 2 teams. Notably, the typical physique weight of the experimental group elevated steadily through the remark interval because the management group, in flip, additional indicating the injection of PEG-Ag2S tremendous NPs doesn’t intrude with regular metabolic processes. Moreover, for long-term biocompatibility evaluation, we carried out a 28-day hematological toxicity evaluation (Fig. 5f-l and Determine S11) and histological evaluations (Determine S12). The outcomes revealed the time-evolution development of ALT, AST, ALP, creatinine (CREA), lactate dehydrogenase (LDH), whole bilirubin (TBIL) and UREA in mice injected with PEG-Ag2S tremendous NPs (100 µL, 0.15 mg/mL) inside 28 days. The focus of all biomarkers remained inside regular physiological ranges all through the research interval. No necrotic or hemorrhagic lesions had been detected in any of the analyzed tissues. Collectively, these findings strongly point out that PEG-Ag2S tremendous NPs are extremely biocompatible and non-toxic, making them a promising candidate for in vivo functions.
Ex vivo and In vivo cytotoxicity efficiency of PEG-Ag2S tremendous NPs. Cell viability of MCF-10 A, 293-T, HepG2 and MCF-7 cell after 24 h (a) and 48 h (b) incubation with completely different concentrations of PEG-Ag2S tremendous NPs within the cell medium as decided by MTT assays. The error bars symbolize three parallelled measurements. Time evolution of meals consumption (c), physique weight (d) and physique temperature (e) of three Kunming mice after intraperitoneal injection of 300 µL of a dispersion of Ag2S tremendous NPs in PBS (0.5 mg/mL, whole dose of 150 µg). The outcomes obtained from three management mice injected with 300 µL PBS are included for a comparability. Serum focus of hepatic enzymes (f, g, and h), creatinine (i), lactate dehydrogenase (j), whole bilirubin (ok) and urea (l) for mice intravenously injected with Ag2S tremendous NPs and management mice as obtained 1 and 28 days after injection (n = 5 for every group), the error bars correspond to the usual error of the imply
Although the PEG-Ag2S tremendous NPs with good biocompatibility demonstrated profitable hydrophilic modification through our method, issues concerning potential fluorescence quenching because of this hydrophilic transformation nonetheless persist. The fluorescence lifetime of PEG-Ag2S tremendous NPs dispersed in PBS reveals a worth close to to 2.1 µs (Determine S13a), indicating that the neither the PEG coating nor dispersion in PBS induces fluorescence quenching within the modified PEG-Ag2S tremendous NPs. This phenomenon, the place the decay time stays steady in each CHCl3 and PBS, once more reveals the protecting functionality of the formatted AgCl shell in opposition to the photon rest course of induced by the vibration of H2O molecules [48]. Consequently, the optimized Ag2S tremendous NPs exhibit comparable QY values in each solvents. For the sake of the comparability, Determine S13a contains the fluorescence decay curve of business PEG-Ag2S NPs dispersed in PBS (obtained from Sinano Corp. China) as nicely. These business NPs exhibit a lifetime worth of 55 ns, which is roughly 36 occasions shorter than that of the PEG-Ag2S tremendous NPs dispersed in PBS. Furthermore, the NIR-II photos of PEG-Ag2S tremendous NPs and PEG-Ag2S NPs dispersed in numerous media (PBS, FBS and DMEM) at a focus of 0.15 mg/mL had been included in Determine S13 as nicely, which reveal the excellent NIR-II imaging functionality of Ag2S tremendous NPs after PEG modification. Consequently, the enlarged lifetime and QY values guarantee the potential of the PEG-Ag2S tremendous NPs for subsequent high-quality, low-dose in vivo NIR-II imaging.
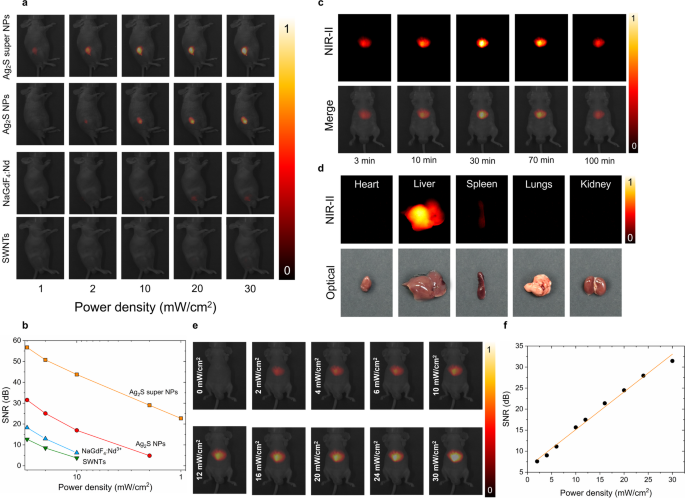
For the sake of the simple analysis of the in vivo efficiency of the Ag2S tremendous NPs, we chosen generally used NIR imaging fluorescent probes, i.e. business Ag2S NPs, neodymium-doped rare-earth fluoride NPs (NaGdF4: Nd) and single-walled nanotubes (SWNTs), for a comparability. 4 teams of 6-week nude mice (n = 3 for every group) had been chosen for the comparability research and subjected to a subcutaneous injection with 100 µL of various PBS options containing Ag2S tremendous NPs, business Ag2S NPs, NaGdF4: Nd and SWNTs at a focus of 1.5 mg/mL, akin to a complete NPs injection dose of 5 mg/kg per mouse. As illustrated in Fig. 6a, the in vivo NIR-II fluorescence photos had been captured for every group below 808 nm-laser irradiation with energy density starting from 1 mW/cm2 to 30 mW/cm2. One may discover that Ag2S tremendous NPs exhibit excellent brightness in comparison with all different chosen NIR probes below an identical experimental circumstances. Primarily based on our earlier investigation, the brightness affected by doable manufacture variation associated to the subcutaneous injection is negligible through the NIR-II imaging remark [39], in flip, the visible NIR emitting efficiency distinction throughout all of the fluorescence probes could be solely attributed to variations in brightness. Thus, the superior brightness of Ag2S tremendous NPs is able to acquiring the NIR sign even at a particularly low laser irradiation energy density of 1 mW/cm2, the place no fluorescence sign is detected from some other chosen NIR probes. The signal-to-noise ratio (SNR) values of various NIR probes are quantified in Fig. 6b, exhibiting the facility density dependence property, which is additional illustrated in NIR-II photos obtained below completely different energy densities after 30 min of intravenous injection (Fig. 6e-f). Furthermore, Fig. 6a reminds us that the synthesized Ag2S tremendous NPs has decreased the minimal excitation energy density over 30 occasions. This method not solely permits in vivo imaging utilizing cost-effective excitation sources but in addition ensures minimal thermal loading throughout picture acquisition, contributed by Ag2S tremendous NPs or the high-power excitation laser (Determine S14).
In Vivo imaging efficiency of PEG-Ag2S tremendous NPs. (a) NIR-II fluorescence photos obtained below the 808 nm laser illumination at completely different energy densities from 4 teams of mice (n = 3 for every group) subcutaneously injected with 100 µL options containing Ag2S tremendous NPs, business Ag2S NPs, NaGdF4: Nd NPs and SWNTs at a focus of 1.5 mg/mL, (b) signal-to-noise ratio (SNR) as a operate of energy density quantified from the evaluation of photos included in (a), (c) time-evolution NIR-II fluorescence photos of the mouse intravenously injected with Ag2S tremendous NPs dispersed in PBS (0.15 mg/mL, 100 µL), (d) NIR-II fluorescence photos of ex vivo organs of coronary heart, liver, spleen, lungs and kidney after 100-min intravenously injected with PEG- Ag2S tremendous NPs (100 µL, 0.15 mg/mL) and corresponding optical pictures, (e) NIR-II fluorescence photos with CD1 mouse intravenously injected with 100 µL PEG-Ag2S tremendous NPs dispersed in PBS at a focus of 0.15 mg/mL, buying below 808 nm laser irradiation at completely different energy density after 30-min injection when the NPs amassed to liver, and corresponding signal-to-noise-ratio (SNB, f)
With such an excellent subcutaneous imaging efficiency of Ag2S tremendous NPs, we subsequently carried out an experiment to watch the in vivo biodistribution of our Ag2S tremendous NPs through intravenous injection (100 µL, 0.15 mg/mL). The entire injection dose (15 µg, ~ 0.5 mg/kg) is multiple order of magnitude smaller than the administered dose (6.6 mg/kg) reported for NIR-II biodistribution measurement with Ag2S NPs [49]. The NIR-II photos in Fig. 6c had been obtained below 808 nm laser at an influence density of 30 mW/cm2, which is way decrease than the protection threshold at 808 nm (329 mW/cm2) established by the Worldwide Fee on Non-ionizing Radiation Safety (ANSI Z136.1-2000) [50]. One may discover that throughout the first 3 min after injection, elements of the PEG-Ag2S tremendous NPs started to build up within the liver with detectable fluorescence indicators protecting a small space. After that, nearly all of PEG-Ag2S tremendous NPs had been captured by the liver after 30 min as a result of filtration by the reticuloendothelial system, which is corroborated by the ex vivo organs NIR-II photos acquired at 100 min after injection (Fig. 6d) and its corresponding quantification outcomes (Determine S15). It must be identified that the fluorescence depth of the liver decreased about 10% at 100 min in comparison with the one recorded at 30 min, which could possibly be attributed to the partial elimination of Ag2S tremendous NPs by Kupffer cells and liver sinusoidal endothelial cells [51].
In vivo focused inflammatory imaging with Ag2S tremendous NPs
Primarily based on the excellent in vivo imaging efficiency of Ag2S tremendous NPs, it’s able to using PEG-Ag2S tremendous NPs for high-quality and low-dose irritation imaging. For the sake of that, we established fashions of myocarditis and gastritis with 4-week-old feminine mice. Earlier analysis has reported that macrophages optimistic for the inflammatory chemokine CXC ligand 9 (CXCL9) represent essentially the most dominant cell kind through the acute part of irritation [52, 53]. For exact concentrating on irritation imaging, a sequence-specific peptide ligand able to selectively binding to CXCL9 was conjugated onto PEG-Ag2S tremendous NPs and business PEG-Ag2S NPs through dehydration synthesis (Experimental part in Supporting Info), in flip, endowing concentrating on skill to Ag2S NPs for infected tissues. By way of zeta potential evaluation (Determine S16) and high-performance liquid chromatography (HPLC) characterization (Determine S17), we assessed the conjugation effectivity of CXCL9-targeted peptides with PEG-Ag2S tremendous NPs. The outcomes demonstrated a major shift in zeta potential, from − 34.6 ± 0.2 mV for PEG-Ag2S tremendous NPs to −5.7 ± 0.3 mV for T-Ag2S tremendous NPs (after peptide functionalization), indicating profitable conjugation of the CXCL9-targeting peptide. Moreover, the HPLC evaluation revealed a retention time of 12 min for the conjugate, which was 8 min shorter than the 20 min retention time of the free peptide, additional confirming the formation of the CXCL9 peptide-conjugated construction.
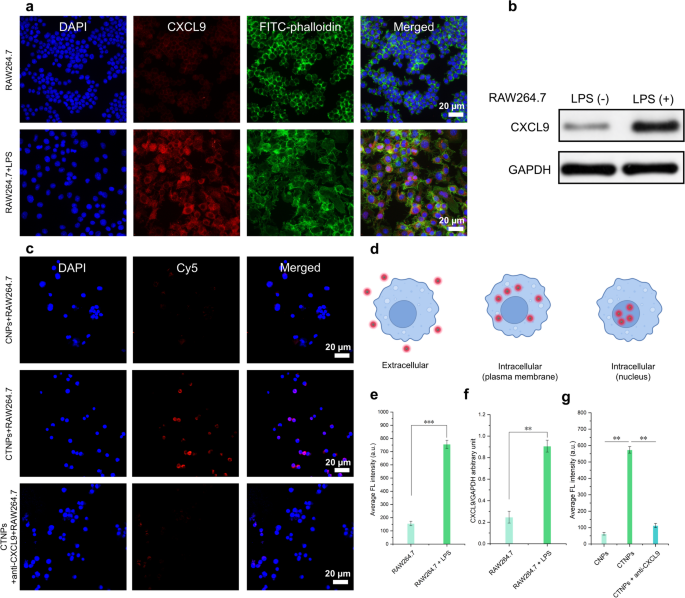
Then, the expression of CXCL9 was additional verified in vitro utilizing lipopolysaccharide (LPS)-induced macrophages. As proven in Fig. 7a, immunofluorescence photos revealed that LPS-treated RAW 264.7 cells, a typical consultant of mouse macrophages, exhibited robust purple fluorescent indicators after incubation with anti-CXCL9 antibodies. To facilitate visualization, the nuclei and cytoskeleton of LPS-treated RAW 264.7 cells had been stained blue and inexperienced, respectively. In distinction, no detectable purple fluorescence was noticed in LPS-untreated macrophages. The numerous distinction in CXCL9 expression between inflammatory macrophages and regular macrophages was additional confirmed by the corresponding statistical evaluation in Fig. 7e. Moreover, western blot evaluation corroborated these findings (Fig. 7b and f). Having assessed the degrees of CXCL9 in LPS-induced macrophages in vitro, we subsequent evaluated the flexibility of T-Ag2S tremendous NPs to focus on CXCL9 in LPS-stimulated RAW 264.7 cells. Determine 7d presents a schematic diagram of cell uptake. Because the confocal laser scanning microscopy (CLSM) instrument we used on this work can’t detect NIR-II fluorescence indicators, Cy5-PEG-NH2-labeled T-Ag2S tremendous NPs (Cy5-T-Ag2S tremendous NPs, CTNPs) and PEG-Ag2S tremendous NPs (Cy5-PEG-Ag2S tremendous NPs, CNPs) had been employed for the next mobile uptake research. The CNPs and CTNPs had been then co-incubated with LPS-induced macrophages to analyze the mobile internalization habits of the NPs. CLSM photos of the CTNPs group revealed distinguished purple fluorescence. Within the CNPs group, nonetheless, solely minimal intracellular purple fluorescence was noticed (Fig. 7c). Furthermore, when LPS-induced macrophages had been pretreated with a CXCL9 antibody, the depth of intracellular purple fluorescence was markedly diminished (Fig. 7g). These findings spotlight the important position of CXCL9 concentrating on in facilitating the efficient binding of nanoprobes to inflammatory cells. Consequently, this means that T-Ag2S tremendous NPs are extra readily phagocytosed by macrophages in comparison with PEG-Ag2S tremendous NPs, doubtless because of enhanced CXCL9-mediated interactions. These outcomes present a stable experimental basis for the applying of inflammation-targeted T-Ag2S tremendous NPs in in vivo inflammatory imaging.
In vitro ranges of CXCL9 in LPS-induced macrophages and analysis of in vitro focused binding of Ag2S tremendous NPs. (a) Immunofluorescence picture of CXCL9 in RAW264.7 cells induced by LPS (100 ng/mL). Blue fluorescence: DAPI; inexperienced fluorescence: FITC-phalloidin; purple fluorescence: CXCL9. (b) Western blot evaluation of CXCL9 expression in RAW264.7 cells. (c) CLSM photos of LPS-induced mouse (RAW264.7) macrophages endocytosis of CNPs, CTNPs, and CTNPs + anti-CXCL9. Blue fluorescence: DAPI; purple fluorescence: Cy5-PEG-NH2-labeled PEG-Ag2S tremendous NPs or T-Ag2S tremendous NPs. CNPs: Cy5-PEG-Ag2S tremendous NPs, and CTNPs: Cy5-T-Ag2S tremendous NPs. (d) Schematic diagram of cell uptake (e) Statistics of CXCL9 expression based mostly on the typical fluorescence depth of (a). (f) Relative worth of CXCL9/GAPDH stripe grey (n = 3). (g) Common fluorescence depth of every group in (c). Knowledge are represented as imply ± SD (n = 3), ** p < 0.01, ***p < 0.001
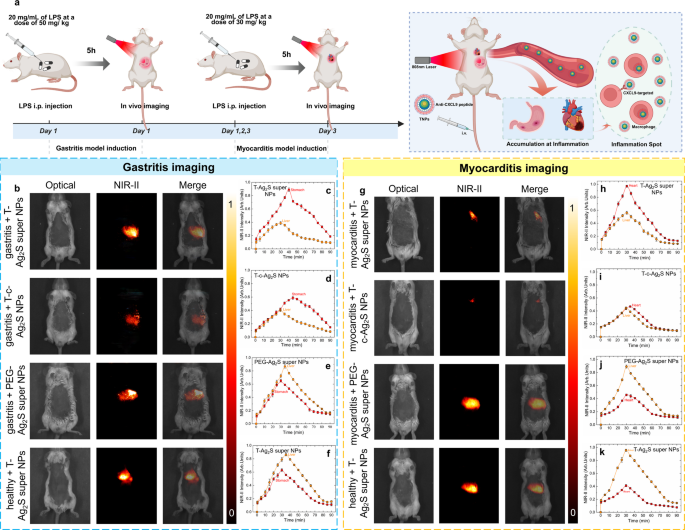
To induce gastritis, lipopolysaccharide (LPS) solvent at a focus of 20 mg/mL (whole dose of fifty mg/kg per mouse) was intraperitoneally injected to the mice after which subjected to a steady monitor physiological standing for five h till the gastritis mannequin was efficiently established. Histopathological and hematological analyses confirmed the profitable institution of the mouse gastritis mannequin (Determine S18). H&E staining of gastric tissues (Determine S18a–b) revealed that, in contrast with the management group, mice within the gastritis group exhibited marked mucosal structural harm and inflammatory cell infiltration. Moreover, serum ranges of inflammatory markers, together with tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), prostaglandin E₂ (PGE₂), C-reactive protein (CRP), and malondialdehyde (MDA) had been considerably elevated (Determine S18c–g), additional supporting the profitable induction of gastritis within the mouse mannequin. To additional current the concentrating on and excellent imaging functionality of the Ag2S tremendous NPs after focused peptide modification (T-Ag2S tremendous NPs), mice had been randomly divided into 4 teams: (1) gastritis + T-Ag2S tremendous NPs; (2) gastritis + T-commercial Ag2S NPs (T-c-Ag2S NPs); (3) gastritis + PEG-Ag2S tremendous NPs; (4) wholesome + T-Ag2S tremendous NPs. The mice in numerous teams had been intravenously injected with corresponding NPs dispersed in PBS (100 µL, 0.15 mg/mL) and carried out steady NIR-II photos acquisition for 90 min (schematic illustration in Fig. 8a). The NIR-II photos included in Fig. 8b had been obtained 35 min after intravenous injection, the entire photos throughout monitoring for every group had been offered in Determine S20-S23 (from 30 s to 90 min). As proven in Fig. 8b, the imaging outcomes reveal a preferential accumulation of targeting-modified Ag2S NPs on the gastritis-affected tissues within the abdomen. It’s value noticing that the time-evolution fluorescence depth of the group injected with T-Ag2S tremendous NPs (Fig. 8c) is considerably brighter than on the gastritis irritation web site in comparison with that of the group injected with T-c-Ag2S NPs (Fig. 8d). The comparability of NIR-II photos of the above two teams and corresponding pixel profile in Determine S24 signifies that the Ag2S tremendous NPs improved the fluorescence sign by 92% in comparison with business Ag2S NPs. Quite the opposite, when utilizing non-targeted PEG-Ag2S tremendous NPs or within the absence of gastritis irritation, NIR-II photos illustrated that the injected NPs had been preferentially amassed to dwell as a result of retention of nanoparticles within the liver (Fig. 8b). The phenomenon is corroborated by the corresponding time-evolution NIR-emitting fluorescence depth (Fig. 8e-f) and ex vivo NIR-II photos and their quantification histograms (Determine S25-S26). The NIR-II fluorescence photos point out the profitable in vivo concentrating on functionality of the T-Ag2S tremendous NPs and T-c-Ag2S NPs to gastritis. Extra importantly, the outcomes additional spotlight the superior in vivo imaging efficiency of the Ag2S tremendous NPs characterised by greater brightness below low-dose injection circumstances in comparison with business Ag2S NPs.
(a) Schematic illustration of in vivo concentrating on inflammatory imaging with Ag2S tremendous NPs, (b) in vivo NIR-II gastritis imaging outcomes with completely different teams (from as much as down): gastritis + T-Ag2S tremendous NPs, gastritis + T-c-Ag2S NPs, gastritis + PEG-Ag2S tremendous NPs, wholesome + T-Ag2S tremendous NPs, and their corresponding time-evolution NIR-II fluorescence depth of abdomen and liver (c-f), (g) in vivo NIR-II myocarditis imaging outcomes with completely different teams (from as much as down): myocarditis + T-Ag2S tremendous NPs, myocarditis + T-c-Ag2S NPs, myocarditis + PEG-Ag2S tremendous NPs, wholesome + T-Ag2S tremendous NPs, and their corresponding time-evolution NIR-II fluorescence depth of coronary heart and liver (h-k)
Primarily based on the above gastritis irritation imaging outcomes, we subsequently performed in vivo myocarditis imaging. To induce myocarditis, LPS solvent at a focus of 20 mg/mL (whole dose of 30 mg/kg per mouse) was intraperitoneally injected to the mouse after which subjected to a steady monitor physiological standing for 3 days till the myocarditis mannequin was efficiently established. The histological and quantitative analyses of inflammatory markers (Determine S19) confirmed the profitable institution of the myocarditis mannequin. Histological photos (Determine S19a–b) revealed that the myocardial tissue within the myocarditis group exhibited marked structural disruption together with intensive inflammatory cell infiltration, in clear distinction to the well-preserved structural integrity noticed within the management group. Furthermore, the measurements of inflammatory markers (Determine S19c–g) confirmed that the degrees of tumor necrosis issue α (TNF-α), cardiac troponin T (cTnT), cardiac troponin I (cTnI), interleukin-6 (IL-6), and creatine kinase isoenzyme (CK-MB) had been considerably elevated within the myocarditis group. These will increase not solely mirror heightened native inflammatory exercise but in addition point out the prevalence of myocardial cell harm, thereby confirming the profitable induction of the myocarditis mannequin in mice. For the sake of vivid and concentrating on imaging, the 4-week feminine mice had been randomly divided into 4 teams: (1) myocarditis + T-Ag2S tremendous NPs; (2) myocarditis + T-commercial Ag2S NPs (T-c-Ag2S NPs); (3) myocarditis + PEG-Ag2S tremendous NPs; (4) wholesome + T-Ag2S tremendous NPs. The mice in every group had been intravenously injected with corresponding NPs dispersed in PBS (100 µL, 0.15 mg/mL) and steady NIR-II imaging was carried out each 5 min for a complete period of 90 min. The NIR-II fluorescence photos included in Fig. 8g for myocarditis imaging had been obtained 30 min after intravenous injection, and the NIR-II photos all through the experimental course of in every group had been offered in Determine S27-S30. As anticipated, the NPs modified with concentrating on peptide, particularly T-Ag2S tremendous NPs and T-c-Ag2S NPs, exhibited preferential accumulation within the infected coronary heart tissues. On the one hand, it’s evident that the brightness of T-Ag2S tremendous NPs improved over 90% than that of T-c-Ag2S NPs as noticed by way of the time-evolution fluorescence depth within the coronary heart (Fig. 8h-i), which is additional supported by the pixel profile included in Determine S31. Alternatively, the NPs with out modification of concentrating on peptide would like to predominantly accumulate to the liver (Fig. 8j-k). NIR-II photos of ex vivo organs and the quantification outcomes included in Determine S32-S33 reveal that the NPs modified with concentrating on peptide accumulate to the liver, coronary heart and lungs, whereas for the wholesome + T-Ag2S tremendous NPs or myocarditis + PEG-Ag2S tremendous NPs teams, the fluorescence sign is simply noticed in liver and spleen. The remark of T-Ag2S tremendous NPs and T-c-Ag2S NPs within the lungs could also be attributed to the dysfunction of the alveolar-capillary barrier, which may probably result in pulmonary congestion. This, in flip, might impair gasoline trade and end in respiratory misery, as beforehand reported [54, 55]. All the outcomes offered above additional revealed the excellent functionality of as-synthesized PEG-Ag2S tremendous NPs for low dose, high-brightness, and high-quality in vivo irritation imaging.