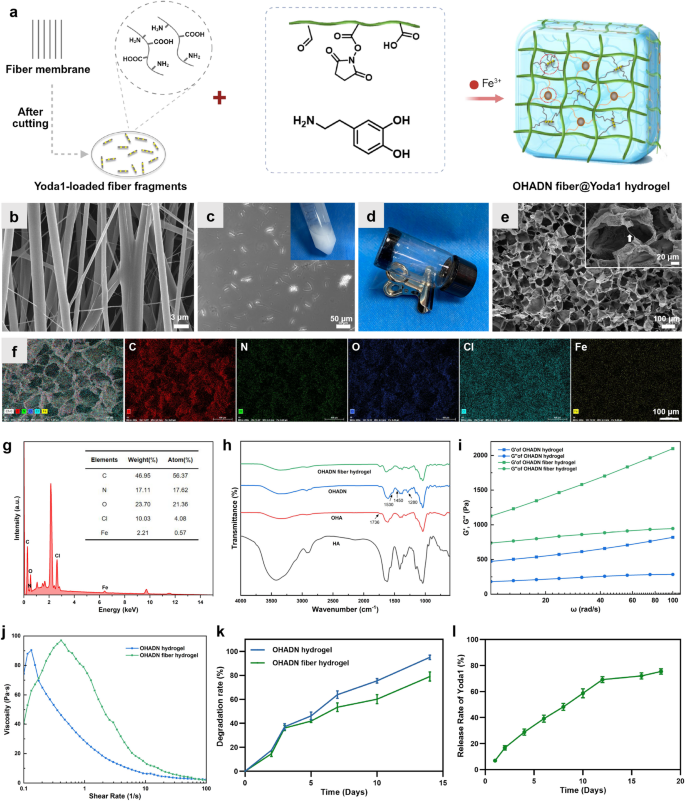
Synthesis and characterization of the OHADN fiber@Yoda1 hydrogel
The OHADN fiber@Yoda1 hydrogel was fabricated utilizing a dual-crosslinking mechanism involving catechol-Fe³⁺ coordination and imine covalent bonds, as proven in Fig. 1a. The oriented fibers loaded with Yoda1 had been ready utilizing the well-established electrospinning approach [19]. SEM evaluation confirmed the uniform alignment of the fibers, as proven in Fig. 1b. This unidirectional orientation facilitates the exact slicing of the fibers into fragments. By controlling the thickness of the cryosectioning, we obtained fiber fragments of constant dimension. The optimized section size was roughly 20–50 μm, guaranteeing their uniform distribution inside the hydrogel matrix with out compromising injectability. As illustrated within the inset of Fig. 1c, a homogeneous translucent emulsion containing the fiber fragments was obtained. Contemplating that the tensile energy of the oriented fibers can attain as much as 16 MPa, these fragments maintain nice potential for enhancing the mechanical properties of injectable hydrogels whereas sustaining their processability.
In biomaterial science, the native extracellular matrix element HA has been strategically repurposed as a multifunctional hydrogel precursor, notably advantageous for minimally invasive supply via injectable formulations on account of its inherent shear-thinning conduct [30]. After oxidation, HA may be functionalized with aldehyde teams on its molecular chains, thereby providing enhanced prospects for the development of its hydrogels. OHA-based hydrogels have gained important consideration as filling supplies for irregular bone defects on account of their distinctive spatial adaptability [21, 22]. The distinctive linear macromolecular structure of OHA contributes to hydrogel moisture retention and elastic properties. On this research, hydrogel formation was confirmed via vial inversion exams, with consultant outcomes proven in Fig. 1d.
The microstructure and elemental composition of the hydrogel had been characterised utilizing SEM and EDS. As proven in Fig. 1e, the OHADN fiber@Yoda1 hydrogel displayed a well-defined porous construction with a comparatively uniform pore dimension distribution. This architectural function is especially helpful for facilitating cell infiltration and nutrient transport, each of that are crucial necessities for profitable tissue engineering functions. Particularly, interconnected fiber fragments had been noticed bridging the hydrogel partitions, which is especially important for sustaining structural integrity throughout degradation. These residual fibers play a vital position in preserving mechanotransduction signaling by sustaining mobile traction forces, thereby creating a good mechanical microenvironment that helps prolonged osteogenic differentiation processes. The EDS elemental mapping evaluation (Fig. 1f and g) revealed that the OHADN fiber@Yoda1 hydrogel primarily consisted of carbon (C), nitrogen (N), oxygen (O), chlorine (Cl), and iron (Fe). The presence of those parts is in keeping with the anticipated composition of the hydrogel system and suggests profitable incorporation of the specified parts.
OHA was synthesized via NaIO4-mediated oxidation of the proximal hydroxyl (-OH) group in HA. FTIR evaluation (Fig. 1h) confirmed the aldehyde group formation in OHA, evidenced by a attribute peak at 1736 cm⁻¹ akin to the -CHO group. Comparative evaluation revealed enhanced OHADN peaks at 1530 cm⁻¹ (C––N stretching), 1450 cm⁻¹ (fragrant C–C stretching), and 1280 cm⁻¹ (C–N stretching) within the arrow-marked areas. These observations point out profitable dopamine grafting onto HA by way of Schiff base formation between the fragrant amine (-NH2) and OHA’s aldehyde group, in keeping with reported mechanisms [29].
The matrix stiffness of hydrogels is a crucial issue influencing cytoskeleton group, cell migration, and differentiation [31, 32]. To research whether or not the incorporation of cross-linked fiber fragments might improve the mechanical properties of OHADN hydrogels, we carried out rheological experiments to guage the storage modulus (G’) and loss modulus (G”). It’s well-established {that a} increased G’ worth in comparison with G” signifies the profitable formation of a steady hydrogel community [29]. As proven in Fig. 1i, each the OHADN hydrogels and the OHADN fiber hydrogels exhibited G’ values that had been persistently increased than their corresponding G” values throughout the frequency vary of 10–100 rad s−1, confirming the formation of steady hydrogel networks. Moreover, the G’ values of the OHADN fiber hydrogels had been considerably increased than these of the OHADN hydrogels, demonstrating that the incorporation of cross-linked fiber fragments successfully enhanced the mechanical energy of the hydrogel. The elevated G’ values within the OHADN fiber hydrogels point out larger resistance to deformation underneath utilized stress, a property that’s important for sustaining structural integrity in dynamic physiological environments. This improved mechanical robustness not solely ensures the hydrogel’s stability throughout mobile actions but additionally higher replicates the mechanical properties of native tissues, which is crucial for supporting cell operate and bone regeneration [7, 33].
The adhesive properties of implanted supplies are essential for guaranteeing the retention of filling supplies in irregular jaw defects. To judge the injectability and adhesion of OHADN fiber hydrogels, we analyzed their viscosity-shear charge curves utilizing a rheometer. As proven in Fig. 1j, the viscosity of the OHADN fiber hydrogels decreased with growing shear charge, demonstrating their shear-thinning conduct and confirming their injectability. This property permits the hydrogel to be simply extruded via a syringe whereas sustaining its structural integrity, as evidenced by its capability to stay in a gel state post-injection (Fig. S1a). Moreover, Fig. S1b illustrates that the OHADN fiber hydrogel exhibited sturdy tissue adhesion, a crucial function for efficient defect filling and retention. It’s value mentioning that the adhesiveness of the OHADN fiber hydrogel may be attributed to its distinctive composition and crosslinking mechanism. The OHA molecular chains, wealthy in aldehyde and catechol teams, together with the coordination of Fe³⁺ ions and the covalent motion of imine bonds, not solely kind a steady hydrogel community construction but additionally endow it with glorious interfacial adhesive properties. This makes the hydrogel system an excellent materials for repairing irregular mandibular defects.
When the hydrogel is injected to fill irregular bone defects, self-healing capability is crucial to make sure the formation of a steady barrier that protects in opposition to microbial invasion [34, 35]. As demonstrated in Fig. S1c, the OHADN fiber hydrogel exhibited exceptional macroscopic self-healing properties. When minimize, the hydrogel quickly healed with out exterior intervention, and the healed hydrogel confirmed no seen cracks and could possibly be lifted intact. That is the results of the mixed motion of twin dynamic chemical bonds. The self-healing functionality not solely ensures structural integrity but additionally enhances the hydrogel’s capability to keep up a protecting barrier in dynamic physiological environments, making it extremely appropriate for functions in bone defect regeneration [36].
To validate the crosslinking impact of fiber fragments in injectable hydrogels, we systematically evaluated the degradation kinetics of OHADN fiber hydrogel. As depicted in Fig. 1ok, OHADN fiber hydrogel and OHADN hydrogel exhibited comparable degradation charges in the course of the preliminary 3-day interval. Nevertheless, a definite divergence emerged from day 5 onward, with the OHADN fiber hydrogel demonstrating a slower degradation profile. By day 14, quantitative evaluation revealed a 16% discount in degradation charge of the OHADN fiber hydrogel in comparison with OHADN hydrogel (p < 0.05). The outcome signifies that the covalent cross-linking community shaped by the fiber fragments inside the hydrogel system successfully retards the degradation kinetics of the hydrogel via a double-network reinforcement construction. Furthermore, this commentary aligns with established findings that cell-mediated hydrogel degradation facilitates mobile spreading and generates mechanical pressure conducive to osteogenesis [7]. Following partial hydrogel degradation, the persistence of slower-degrading fiber fragments establishes anchorage platforms supporting cell migration, microenvironments enhancing mobile proliferation and topographic steering for collagen matrix group [26].
Enzymatic degradation experiment was carried out to investigate the discharge profile as proven in Fig. 1l. Throughout hydrogel degradation (1–12 days), Yoda1 exhibited a launch charge slope of 5.66%, which decreased to 0.53% post-hydrogel degradation, indicating that the drug-loaded fibrous fragments, when integrated into the hydrogel matrix, exhibit a wonderful sustained-release impact. Earlier research show electrospun fiber degradation durations as much as 60 days [19]. The hydrogel part initially promotes stem cell migration and spreading via speedy stress leisure, whereas the Yoda1-loaded fiber community subsequently sustains mechanical pressure and stabilizes PIEZO1 channels by way of sustained Yoda1 launch to boost osteogenic differentiation. Analysis confirms Yoda1, a low-molecular-weight compound, selectively prompts PIEZO1 by stabilizing its open conformation and lowering mechanical activation thresholds [37]. Single-channel kinetic evaluation reveals Yoda1-induced conformational modifications: long-closed state occupancy decreased from 89.6 to 74.6%, long-open state elevated from 7.3 to 22.5%, with slowed inactivation kinetics and accelerated restoration charges, finally attaining 2–3 instances increased open likelihood [18]. These physicochemical properties collectively emphasize the crucial for systematic mobile investigations to validate the therapeutic potential of this hydrogel scaffold system in guiding stem cell destiny.
Synthesis and characterization of the OHADN fiber@Yoda1 hydrogel. (a) Schematic illustration for fabricating the OHADN fiber@Yoda1 hydrogel. (b) SEM picture of aligned fiber fabricating by electrospinning approach. (c) The picture of fiber fragments, which the insets was PBS containing fiber fragments. (d) Vial inversion exams. (e) SEM picture of OHADN fiber@Yoda1 hydrogel. The white arrows denote the fiber fragments (insets). (f and g) EDS elemental evaluation of OHADN fiber@Yoda1 hydrogel. (h) FTIR spectra. (i) The storage modulus (G’) and loss modulus (G’’). (j) The viscosity-shear charge curves. (ok) Degradation experiment. (l) The Yoda1 launch curve
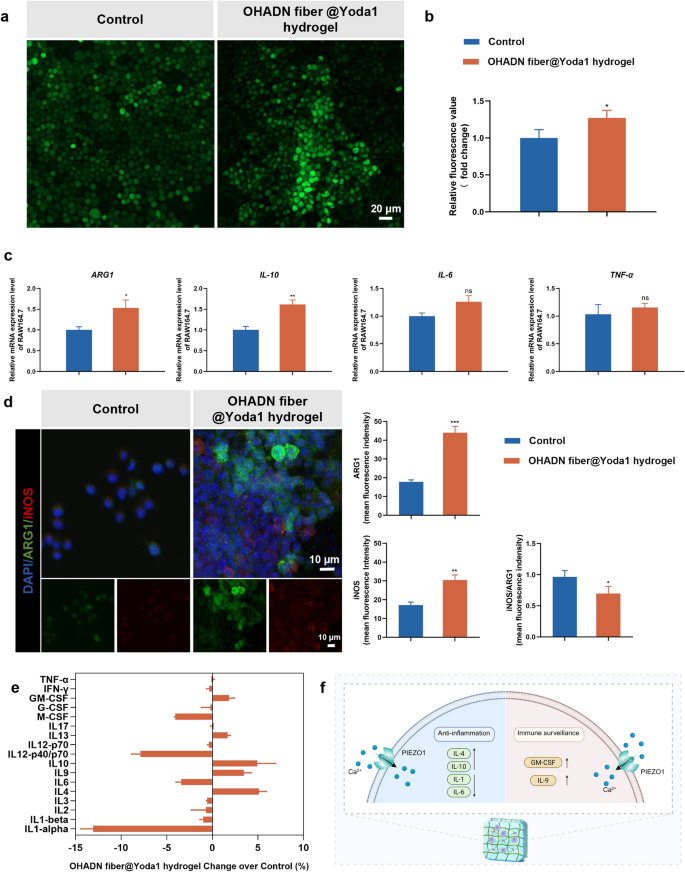
Impact of OHADN fiber@Yoda1 hydrogel on immune microenvironment
Macrophages, as versatile mobile parts of the innate immune system, possess the exceptional capability to dynamically reply to microenvironmental alterations and endure polarization into numerous useful phenotypes, taking part in a pivotal position in tissue restore processes [38]. Mechanical stimuli, notably matrix stiffness, have been demonstrated to advertise the pro-inflammatory activation of macrophages, thereby facilitating the secretion of wound-healing cytokines that contribute to tissue regeneration [39]. Nevertheless, extreme matrix stiffness could set off extreme overseas physique responses and result in fibrous capsule formation [40]. Consequently, it’s crucial to systematically consider the inflammatory responses elicited by biomaterials on the mobile degree.
Rising proof has demonstrated that PIEZO1 channel-mediated calcium inflow performs a regulatory position in macrophage inflammatory responses and therapeutic processes [40]. To research this mechanotransduction pathway, we employed calcium-sensitive fluorescent probes to quantify intracellular calcium ranges in macrophages cultured on OHADN fiber@Yoda1 hydrogel group. As illustrated in Fig. 2a and b, each immunofluorescence imaging and microplate reader analyses persistently revealed a average elevation in intracellular calcium focus in macrophages cultured on OHADN fiber@Yoda1 hydrogel in comparison with management group.
To additional characterize the macrophage polarization state, we carried out complete evaluation of inflammatory cytokine profiles at each transcriptional and secretory ranges. RT-qPCR evaluation (Fig. 2c) demonstrated statistically important upregulation of anti-inflammatory genes ARG1 (P < 0.05) and IL-10 (P < 0.01) within the OHADN fiber@Yoda1 hydrogel group, whereas sustaining comparable ranges of pro-inflammatory genes IL-6 and TNF-α relative to regulate. Immunofluorescence outcomes (Fig. 2d) revealed increased depth of each ARG1 and iNOS within the OHADN fiber@Yoda1 hydrogel group in comparison with the management group; nonetheless, the iNOS/ARG1 ratio was considerably decrease, indicating that the hydrogel induces a reparative microenvironment. Moreover, parallel evaluation of secreted cytokines revealed a definite sample: substantial enhancement of Interleukin-4 (IL-4) and IL-10 manufacturing accompanied by important suppression of a number of pro-inflammatory mediators, notably Interleukin-1 (IL-1) and IL-6, although with a modest enhance in Granulocyte-macrophage Colony-stimulating Issue (GM-CSF) and Interleukin-9 (IL-9) ranges (Fig. 2e).
These outcomes recommend that OHADN fiber@Yoda1 hydrogel promotes a hybrid macrophage phenotype that mixes regenerative anti-inflammatory properties with important immune surveillance features, doubtlessly making a balanced microenvironment conducive to tissue restore whereas sustaining needed immune responsiveness, as proven within the schematic diagram in Fig. 2f. These experimental observations align with the mechanistic insights supplied by Wang et al., whose work elucidated that PIEZO1-mediated mechanotransduction not solely enhances macrophage phagocytic effectivity and apoptotic cell clearance capability but additionally orchestrates a phenotypic shift from pro-inflammatory to anti-inflammatory states via exact regulation of inflammatory marker expression [39].
Impact of OHADN fiber@Yoda1 hydrogel on immune microenviroment. (a) Immunofluorescence imaging of intracellular calcium ion staining. (b) Relative fluorescence worth of intracellular calcium ion detecting by microplate reader. (c) Quantitative RT-PCR evaluation of inflammatory cytokine. (d) Immunofluorescence and semi-quantitative evaluation, crimson for iNOS, inexperienced for ARG1, and blue for nuclei. (e) Inflammatory components concentrations in tradition medium supernatant. (f) The schematic diagram of hybrid macrophage phenotype selling by OHADN fiber@Yoda1 hydrogel. (*P<0.05, **P<0.01, ***P<0.001, ns: not important, by Scholar’s t-test)
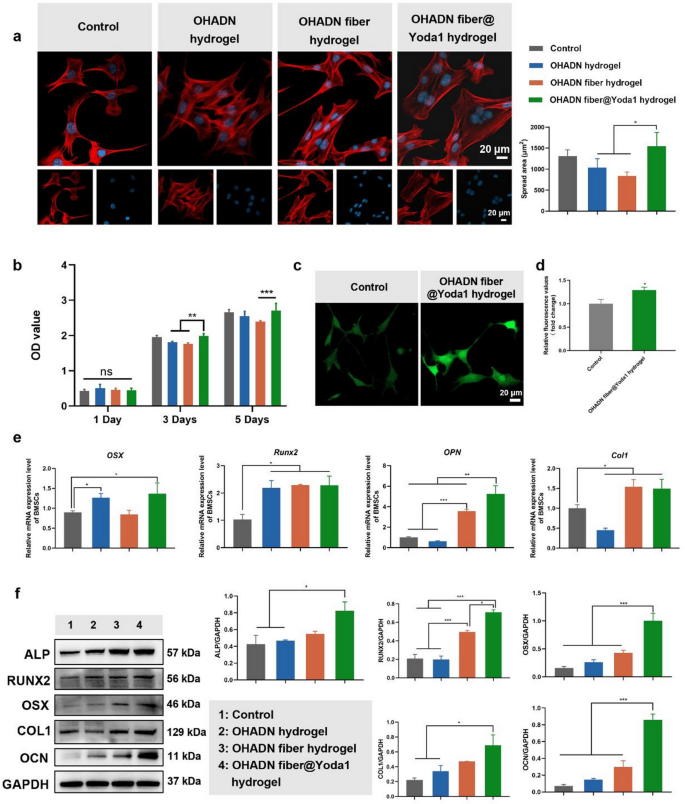
Impact of OHADN fiber@Yoda1 hydrogel on cytocompatibility
The cytocompatibility of biomaterials is essential for his or her scientific software [41]. To judge the biocompatibility of the hydrogels, BMSCs had been seeded onto the hydrogel surfaces, and cell morphology was noticed via cytoskeletal staining, whereas cell viability was assessed utilizing the CCK-8 assay.
As proven in Fig. 3a, cells in all teams exhibited wholesome morphological traits. Notably, cells cultured on the OHADN fiber@Yoda1 hydrogel displayed a bigger spreading space in comparison with these on the OHADN hydrogel and OHADN fiber hydrogel, with no statistically important distinction noticed relative to the management group. The CCK-8 outcomes revealed no important variations in OD values among the many teams on day 1 (Fig. 3b). By day 3 and 5, the OD worth of the OHADN fiber@Yoda1 hydrogel group was increased than that of the OHADN fiber hydrogel teams, with no important distinction in comparison with the management group. These outcomes collectively show that the OHADN fiber@Yoda1 hydrogel reveals glorious cytocompatibility.
The spreading space of cells on biomaterial surfaces is carefully linked to the institution and upkeep of mobile tensional homeostasis, a key issue regulating cell conduct and performance [10]. When cells adhere to a substrate, they generate cytoskeletal pressure via the formation of focal adhesions and actin stress fibers, that are important for sustaining mechanical equilibrium inside the cell [42]. The extent of cell spreading is instantly influenced by the mechanical properties and floor traits of the biomaterial, reminiscent of stiffness, topography, and ligand density [31, 43]. These components collectively decide the flexibility of cells to sense and reply to their microenvironment, a course of often known as mechanotransduction [44].
Within the context of the OHADN fiber@Yoda1 hydrogel, the noticed enhance in cell spreading space means that this materials gives an excellent mechanical microenvironment for cells to ascertain tensional homeostasis. The hydrogel’s composition and structural properties doubtless promote the formation of steady focal adhesions and actin stress fibers, enabling cells to generate and maintain the mandatory cytoskeletal pressure. This, in flip, helps mobile features reminiscent of proliferation, differentiation, and migration, whereas enhancing the general biocompatibility of the fabric.
Moreover, the position of mechanosensitive ion channels, notably PIEZO1, can’t be neglected on this course of. PIEZO1 channels are instantly gated by membrane pressure and play a vital position in cytoskeletal reorganization and tissue homeostasis, appearing as key sensors of mechanical equilibrium [15]. The low-molecular-weight compound Yoda1, which selectively targets PIEZO1 channels, enhances their sensitivity to mechanical stimuli [37]. Within the OHADN fiber@Yoda1 hydrogel, the incorporation of Yoda1 could additional amplify the mechanotransduction alerts, selling a extra strong mobile response to the mechanical microenvironment supplied by the hydrogel.
OHADN fiber@Yoda1 hydrogel possesses glorious cytocompatibility and osteogenic potential. (a) Cytoskeletal staining and cell-spread space evaluation, blue for nuclei and crimson for F-actin. (b) The cytotoxicity of every group of supplies was assessed by the CCK-8 assay. (c) Immunofluorescence imaging of intracellular calcium ion staining. (d) Relative fluorescence worth of intracellular calcium ion detecting by microplate reader. (e) Quantitative RT-PCR evaluation of osteogenic genes. (f) Western blot evaluation of osteogenic proteins. (*P<0.05, **P<0.01, ***P<0.001, ns: not important. The determine d was analyzed by Scholar’s t-test, whereas the remaining figures had been analyzed utilizing one-way ANOVA, adopted by a LSD take a look at.)
OHADN fiber@Yoda1 hydrogel possesses glorious osteogenic potential
After confirming passable cytocompatibility, BMSCs had been seeded onto the surfaces of varied supplies and subjected to osteogenic induction for 7 days. Intracellular calcium ranges had been decided by staining with the fluo-4 probe as proven in Fig. 3c and d. The expression of osteogenic genes and proteins was evaluated utilizing RT-qPCR, western blot and immunofluorescence staining, as proven in Fig. 3e and f and S2.
Intracellular calcium ion staining revealed that the OHADN fiber@Yoda1 hydrogel group exhibited a considerably increased fluorescence depth in comparison with the management group, suggesting enhanced calcium inflow mediated by this hydrogel. This phenomenon is presumably attributed to the activation of the mechanosensitive PIEZO1 ion channel, which is particularly potentiated by the Yoda1 agonist integrated within the hydrogel system. As a prototypical mechanosensitive ion channel, PIEZO1 coordinates mechanochemical transduction by detecting plasma membrane pressure variations [18]. This structural sensor initiates calcium ion inflow via its attribute trimeric propeller configuration, subsequently triggering mechanotransduction cascades involving Hippo pathway effectors YAP/TAZ+ [14]. The nuclear translocation of those transcriptional co-activators basically governs the osteodifferentiation dedication of mesenchymal stem cells [17, 45, 46].
RT-qPCR outcomes revealed that the OHADN fiber@Yoda1 hydrogel group exhibited elevated expression of osteogenic genes, together with OSX, Runx2, OPN, and Col1, in comparison with the management group. Nevertheless, the variations in osteogenic gene expression ranges among the many materials teams had been inconsistent. Particularly, Runx2 expression was increased in all materials teams in comparison with the management, with no important variations noticed between the fabric teams. The expression of OSX was considerably elevated within the OHADN hydrogel and OHADN fiber@Yoda1 hydrogel teams in comparison with the management, whereas the OHADN fiber hydrogel group confirmed no important distinction relative to the management. Western blot outcomes additional supported these findings, displaying that the OHADN fiber@Yoda1 hydrogel group exhibited increased protein ranges of ALP, RUNX2, OSX, COL1, and OCN in comparison with the management group, in keeping with the RT-qPCR information. Moreover, immunofluorescence staining demonstrated that the expression of RUNX2 within the OHADN fiber@Yoda1 hydrogel group was considerably increased than within the different three teams (Fig. S2). These outcomes collectively point out that the OHADN fiber@Yoda1 hydrogel possesses glorious osteogenic potential, successfully selling osteoblast differentiation and maturation.
The noticed upregulation of osteogenic genes and proteins within the OHADN fiber@Yoda1 hydrogel group highlights its superior capability to help osteogenic differentiation. RUNX2, a grasp regulator of early osteogenesis [47], was persistently elevated throughout all materials teams, suggesting that modifications in extracellular matrix stiffness universally affect RUNX2 expression. Nevertheless, the differential expression of OSX, a downstream transcription issue crucial for osteoblast maturation [47], signifies that not all supplies can maintain the development of osteogenic differentiation. The numerous upregulation of OSX within the OHADN fiber@Yoda1 hydrogel group means that this materials gives a conducive microenvironment for superior osteogenic maturation.
The position of mechanotransduction in osteogenesis is additional underscored by the involvement of PIEZO1 channels, that are recognized to mediate mechanical alerts throughout bone growth [13, 14]. Activation of PIEZO1 channels triggers cytoskeletal reorganization, which is crucial for sustaining mobile tensional homeostasis and selling bone regeneration. Our earlier research demonstrated that low-concentration Yoda1, a PIEZO1 activator, upregulates cytoskeleton-related genes and enhances osteogenic protein expression [19]. This aligns with the present findings, the place the OHADN fiber@Yoda1 hydrogel, doubtless via PIEZO1-mediated mechanotransduction, promotes a strong osteogenic response.
The OHADN fiber@Yoda1 hydrogel not solely enhances early osteogenic markers like RUNX2 but additionally helps the expression of late-stage markers reminiscent of OSX and OCN, indicating its potential to drive full osteogenic differentiation. The combination of PIEZO1-mediated mechanotransduction additional amplifies its osteoinductive properties, making it a promising candidate for bone tissue engineering functions.
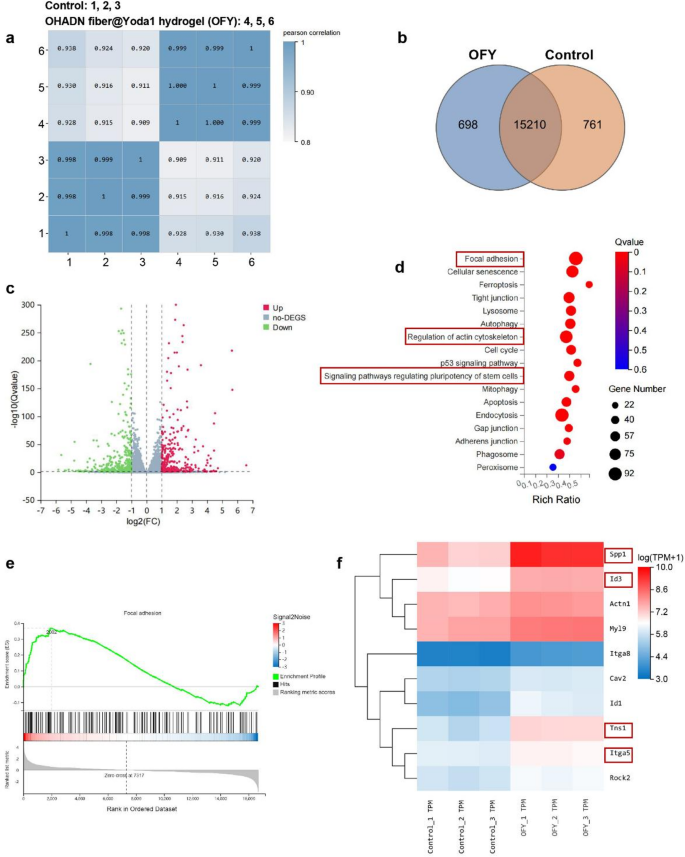
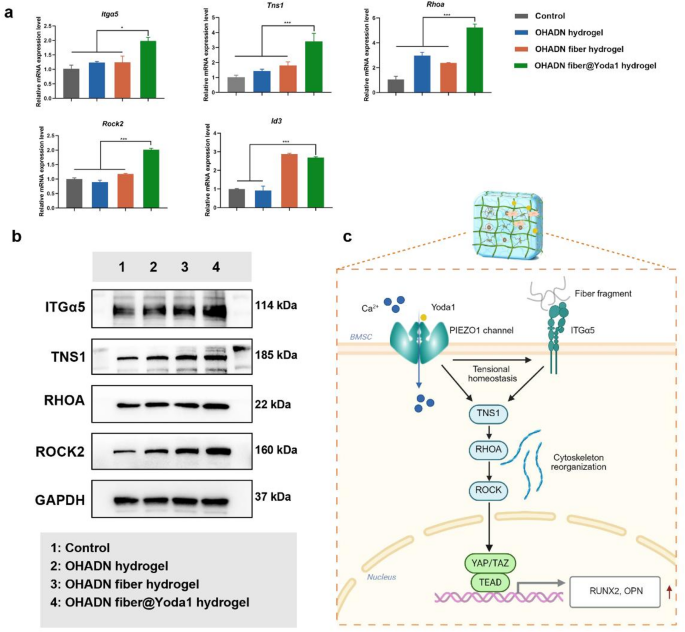
OHADN fiber@Yoda1 hydrogel promotes bone regeneration by way of PIEZO1 – ITGα5 axis
To elucidate the molecular mechanisms underlying OHADN fiber@Yoda1 hydrogel-enhanced osteogenic differentiation of BMSCs, we carried out RNA sequencing evaluation. Excessive-throughput transcriptome sequencing was carried out utilizing the DNBSEQ platform, yielding a median of 6.44 GB clear information per pattern throughout management and OHADN fiber@Yoda1 hydrogel teams. The alignment charges reached 98.96% for reference genomes and 80.38% for annotated gene units, with 16,671 genes detected in complete.
To evaluate the inter-sample correlations in gene expression, Pearson correlation coefficients of all gene expression ranges between each two samples had been calculated. These coefficients had been then visualized in a heatmap (Fig. 4a), with nearly all of correlation coefficients falling inside acceptable limits. Venn diagram evaluation recognized 761 and 698 distinctive differentially expressed genes (DEGs) in management and OHADN fiber@Yoda1 hydrogel teams, respectively (Fig. 4b). Volcano plot evaluation revealed 2,226 upregulated and a couple of,343 downregulated genes with statistical significance (Fig. 4c). Hierarchical clustering demonstrated distinct separation of expression patterns between teams. Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment evaluation of DEGs highlighted important enrichment in focal adhesion, regulation of actin cytoskeleton, and stem cell pluripotency-related signaling pathways within the hydrogel group (Fig. 4d). Gene Set Enrichment Evaluation (GSEA) additional confirmed substantial enrichment of the focal adhesion pathway (NES = 1.60, P < 0.05) in OHADN fiber@Yoda1 hydrogel-treated samples versus controls (Fig. 4e), suggesting its potential involvement in osteogenic differentiation.
Notably, the OHADN fiber@Yoda1 hydrogel group exhibited important upregulation of key osteogenic regulators inside the focal adhesion pathway—Itgα5, Tns1, Spp1 (encoding OPN), and Id3 (a BMP signaling effector). This coordinated gene activation profile (Fig. 4f) reveals a mechanotransduction cascade the place Yoda1-induced PIEZO1 activation primes mechanical signaling via the ITGα5-TNS1 axis, subsequently amplifying mineralization drivers (Spp1/OPN) and activating BMP-responsive transcription components (Id3) to potentiate bone formation. RT-qPCR evaluation demonstrated considerably upregulated mRNA expression ranges of Itgα5, Tns1, Rhoa, Rock2, and Id3 within the OHADN fiber@Yoda1 hydrogel group in comparison with management teams (Fig. 5a). Western blot ends in Fig. 5b and Fig. S3 additional revealed that the OHADN fiber@Yoda1 hydrogel markedly enhanced protein expression of ITGα5, TNS1, RHOA, and ROCK2. These collective findings present compelling proof for the hydrogel’s twin useful mechanism, which concurrently prompts mechanosensitive ion channels and orchestrates downstream osteogenic signaling pathways via spatiotemporal coordination. The schematic diagram in Fig. 5c delineates this advanced working mechanism, highlighting the fabric’s functionality to synchronize mechanical sign notion with biochemical pathway activation.
Transmembrane communication performs a crucial position in responding to extracellular stimuli and sustaining intracellular homeostasis [48, 49]. The PIEZO1 channel serves as a key homeostatic sensor in shaping tissue homeostasis [15, 16]. After disrupting actin filaments with cytochalasin D, the present amplitude of PIEZO1 channels decreases, indicating that the cytoskeleton is liable for transmitting mechanical stimuli upon PIEZO1 channel activation [15]. Atomic pressure microscopy colocalization experiments additional reveal a robust interplay between PIEZO1 channels and the cytoskeleton [50]. The operate of PIEZO1 channels extends past transient transduction of mechanical alerts; they obtain long-term homeostatic steadiness within the tissue microenvironment by dynamically regulating mobile pressure, metabolic exercise, and intercellular communication.
Then again, ITGα5, a core receptor in cell-matrix interactions, transmits mechanical alerts via focal adhesion complexes, whereas tensin regulates cytoskeletal reorganization. These two parts synergistically keep pressure homeostasis [51]. This means that PIEZO1 channels and the integrin system could exhibit cooperative regulatory mechanisms. As the first mechanical sensor of the extracellular matrix, integrin signaling and the mechanical response of PIEZO1 channels are extremely coupled in each spatial and temporal dimensions. Current research have revealed that PIEZO1 channels and integrin β1 on cardiac fibroblast surfaces mutually activate to kind a constructive suggestions loop [52, 53]. Stiffer extracellular matrices upregulate PIEZO1 channel exercise by way of integrin β1 [54], implying that integrins could not directly modulate PIEZO1 channel exercise by regulating cytoskeletal pressure.
v
OHADN fiber@Yoda1 hydrogel promotes bone regeneration by way of PIEZO1 – ITGα5 axis. (a) Quantitative RT-PCR evaluation of Itgα5, Tns1, Rhoa, Rock2, Id3. (b) Western blot assay of ITGα5, TNS1, RHOA, and ROCK2. (c) Schematic of OHADN fiber@Yoda1 hydrogel promoted bone regeneration via the PIEZO1 – ITGα5 axis. (*P<0.05, **P<0.01, ***P<0.001, analyzed by one-way ANOVA, adopted by the LSD take a look at.)
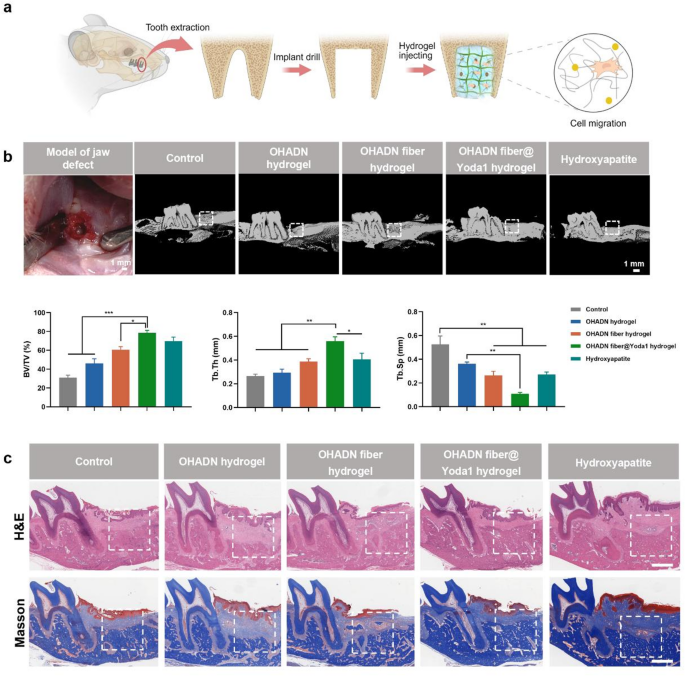
OHADN fiber@Yoda1 hydrogel promoted alveolar bone regeneration in vivo
To research the in vivo osteogenic efficiency of OHADN fiber@Yoda1 hydrogel, we established a rat alveolar bone defect mannequin [55, 56] and implanted experimental supplies into the bone defects (Fig. 6a). Bone regeneration efficacy was systematically evaluated utilizing Micro-CT, H&E staining, Masson’s trichrome staining, and immunohistochemical analyses. Given the well-documented osteoinductive properties of hydroxyapatite, a cloth extensively adopted in bone substitute functions, it was chosen because the constructive management [57, 58]. A clean management group with out materials implantation was included for baseline comparability.
Preliminary biocompatibility evaluation via H&E staining of visceral organs (coronary heart, liver, spleen, lungs, kidneys) revealed no discernible pathological alterations in any experimental group after 4-week implantation, confirming the absence of systemic toxicity and additional validating the biosafety profile of OHADN fiber@Yoda1 hydrogel (Fig. S3).
Micro-CT evaluation with three-dimensional reconstruction (Fig. 6b) demonstrated superior bone regeneration metrics within the OHADN fiber@Yoda1 hydrogel group. Particularly, this group exhibited a considerably increased bone quantity fraction (BV/TV) in comparison with the clean management, OHADN hydrogel, and OHADN fiber hydrogel teams (P < 0.05), whereas displaying comparable efficiency to the Hydroxyapatite constructive management (P > 0.05). The same development was noticed in Tb.Th measurements, suggesting enhanced osteogenic capability. Notably, the OHADN fiber@Yoda1 hydrogel group displayed considerably decrease Tb.Sp than the clean management and OHADN hydrogel teams (P < 0.05), with no statistical distinction from both the OHADN fiber hydrogel or Hydroxyapatite group (P > 0.05). The lowered Tb.Sp noticed within the experimental group aligns with earlier reviews that interconnected fiber networks in composite hydrogels can information osteoblast migration and improve matrix mineralization. These findings collectively point out superior high quality of neoformed bone within the OHADN fiber@Yoda1 hydrogel group.
Constant histological observations had been obtained via H&E and Masson’s trichrome staining analyses (Fig. 6c). Evaluation of newly shaped bone tissue inside the demarcated areas (white dashed traces) demonstrated superior bone formation within the OHADN fiber@Yoda1 hydrogel group in comparison with different experimental cohorts. These findings validate the excellent osteogenic potential and structural steering capabilities of the OHADN fiber@Yoda1 hydrogel.
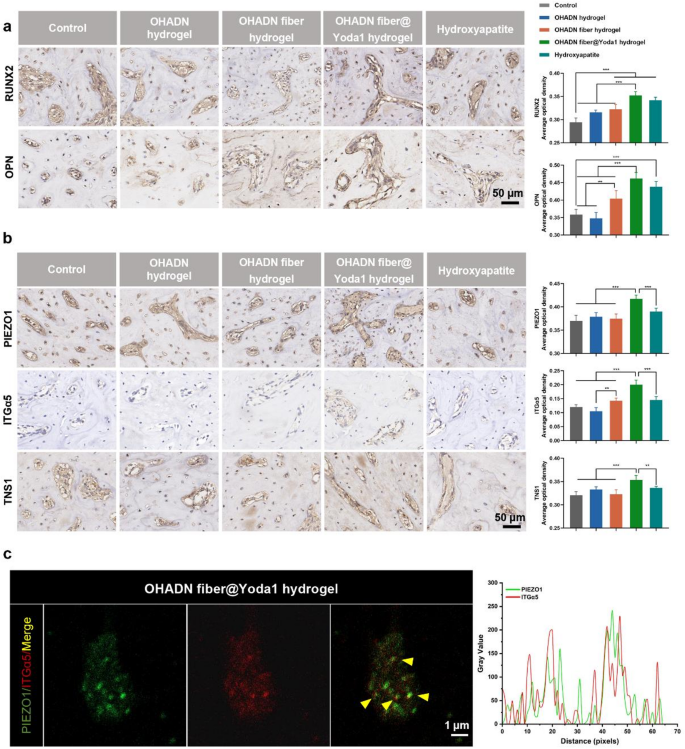
We additional carried out immunohistochemical evaluation to guage the expression ranges of osteogenic proteins and signaling pathway parts. As proven in Fig. 7a, the OHADN fiber@Yoda1 hydrogel group demonstrated considerably increased RUNX2 and OPN expression in comparison with management, OHADN hydrogel, and OHADN fiber hydrogel teams, whereas displaying comparable ranges to the Hydroxyapatite group. Determine 7b revealed that PIEZO1, ITGα5, and TNS1 expression within the OHADN fiber@Yoda1 hydrogel group markedly exceeded these in all different teams. Importantly, immunohistochemical co-staining evaluation of OHADN fiber@Yoda1 hydrogel group demonstrated spatial co-localization of PIEZO1 and ITGα5 (Pearson correlation coefficient = 0.51, Fig. 7c), supporting their useful interplay in mechanotransduction.
The noticed upregulation of PIEZO1 expression could also be attributed to Yoda1-induced activation of PIEZO1 channels via conformational modifications, subsequently triggering elevated ITGα5 and TNS1 expression which in flip regulates PIEZO1 channel expression, suggesting a possible constructive suggestions loop. This discovering signifies a potential synergistic regulatory mechanism between PIEZO1 channels and the integrin system, which is structurally underpinned by their co-localization at focal adhesions. This mechanism is additional supported by proof of mechanical coupling: PIEZO1 colocalizes with integrins, and stiff matrices improve PIEZO1 exercise by way of integrin β1-mediated cytoskeletal pressure [50, 52,53,54]. Current discovering additionally confirmed that PIEZO1 bodily localizes to focal adhesions to manage integrin-FAK signaling meeting [59]. Particularly, PIEZO1 knockdown impairs focal adhesion formation and abrogates integrin-FAK pathway activation, confirming its important position in mechanosensitive adhesion complexes.
Leveraging these cooperative mechanisms, the OHADN fiber@Yoda1 hydrogel achieves spatiotemporal management of mechanosignaling via its biphasic structure: the preliminary hydrogel part facilitates stem cell migration by way of speedy stress leisure, whereas the fiber community sustains mechanical pressure and gives managed Yoda1 launch to stabilize the PIEZO1-ITGα5 signaling hub. Yoda1 sensitizes PIEZO1, enabling Ca²⁺ inflow akin to inflexible substrates regardless of the hydrogel’s sub-rigid properties [18]. This calcium inflow prompts RHOA/ROCK-mediated cytoskeletal contraction [60], elevating ITGα5 expression and enhancing collagen binding for osteogenic differentiation [61]. Collectively, our findings show that the OHADN fiber@Yoda1 hydrogel successfully promotes alveolar bone regeneration via the PIEZO1-ITGα5 mechanosignaling axis. Whereas our information recommend potential crosstalk via membrane deformation when these mechanosensors are in proximity, we acknowledge that absolutely elucidating the direct molecular interaction inside the PIEZO1-ITGα5 axis stays a limitation requiring additional mechanistic validation in ongoing research.
OHADN fiber@Yoda1 hydrogel promoted alveolar bone regeneration in vivo. (a) A schematic diagram of the animal experiment. (b) The 3D reconstruction of micro-CT and evaluation of bone quantity fraction (BV/TV), trabecular thickness (Tb.Th) and trabecular separation (Tb.Sp). (c) Consultant H&E and Masson’s trichrome staining photographs of varied group at week 4. The white dashed field represents the defect location. Scale bars symbolize 1 mm. (**P<0.01, ***P<0.001, analyzed by one-way ANOVA, adopted by the LSD take a look at.)
OHADN fiber@Yoda1 hydrogel promoted alveolar bone regeneration in vivo. (a) Immunohistochemical staining and quantification of common optical density for RUNX2 and OPN of the alveolar bone defect. (b) Immunohistochemical staining and quantification of common optical density for PIEZO1, ITGα5 and TNS1 of the alveolar bone defect. (c) Immunohistochemical co-staining of PIEZO1 (inexperienced) and ITGα5 (crimson). Yellow signifies their spatial co-localization. Proper panel: Quantitative curve of the fluorescence assay analyzed by Picture J software program. (**P<0.01, ***P<0.001, analyzed by one-way ANOVA, adopted by the LSD take a look at.)