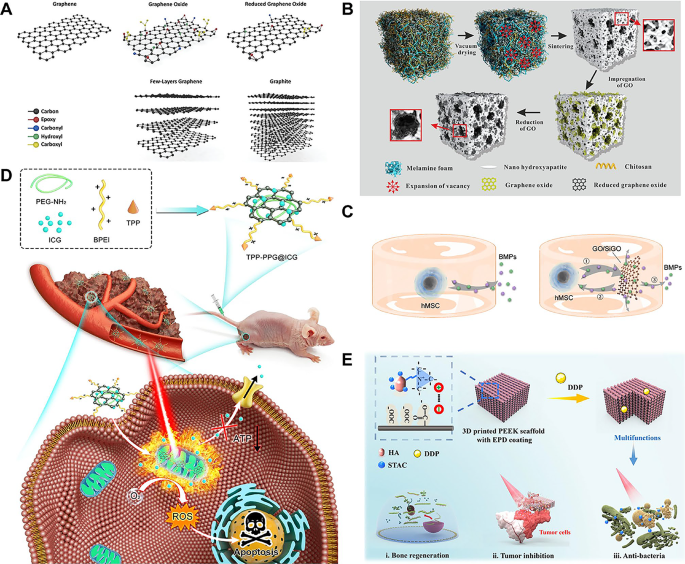
Graphene-based nanomaterials
Graphene is a single layer of carbon the place atoms are packed by covalent sp2 bonds in a hexagonal honeycomb lattice [9]. The ultra-thin thickness and 2D morphological options give it an ultra-high particular floor space and distinctive physicochemical properties. As an allotrope of carbon, graphene possesses acceptable biocompatibility, wonderful in-plane stiffness, distinctive antibacterial traits, and a large open floor able to non-covalent interplay with biomolecules (Fig. 1A) [63,64,65]. Graphene is the primary and most well-known 2D nanomaterial all through the world. Numerous functionalized types of graphene-based nanomaterials (GBN) have been developed and completely investigated, primarily together with graphene, graphene oxide (GO) and decreased graphene oxide (rGO) [66].
As an oxidized type of graphene, GO binds to oxygen-containing purposeful teams overhanging above or under the aircraft of nanomaterials by means of sp3 bonds, together with carboxyl and carbonyl teams on the margins, and hydroxyl and epoxy teams on the basal floor [67]. This chemical modification permits GO extra uneven than graphene, offering extra energetic websites for covalent, electrostatic and hydrogen bond interactions with biomolecules. On the identical time, the presence of oxygen additionally makes GO extra hydrophilic than graphene. rGO is a structural intermediate of graphene and GO that may be readily obtained by thermal, chemical or ultraviolet photoreduction of GO [68]. Compared to GO, rGO comprises a decrease oxygen focus, floor cost and hydrophilic purposeful teams, showing extra hydrophobic [69].
Bone tissue engineering (BTE) has nice potential in treating important bone deficiencies ensuing from extreme trauma and nonunion of fractures. Standard supplies, like metals, ceramics and polymers, have been utilized extensively as BTE scaffolds. Nevertheless, their biocompatibility and osseointegration are insufficient, and it’s difficult to strike a stability between mechanical energy and biodegradation [70]. With the speedy growth of 2D nanomaterials, exceptional biocompatibility, low toxicity and ultra-high particular floor space lead to GBN a perfect organic scaffold for the supply of development components and osteogenic drugs [71]. As well as, the tough floor of GBN and the distinctive non-covalent π-π packing interplay additionally favor the focus of osteogenic media corresponding to dexamethasone and β-glycerophosphate on the nanoplatform, thereby mediating stem cell adhesion, diffusion, proliferation, cell signaling and osteogenic differentiation [72]. Analysis has demonstrated that mesenchymal stem cells (MSCs) derived from various origins exhibit comparable osteogenic tendencies on the floor of hypoxic graphene, and notable activation of angiogenesis components and osteogenic transcription genes might be noticed [73]. The carbon association of graphene intently mimics the microenvironment of the natural bone extracellular matrix (ECM), enabling stem cells to stick, proliferate, and differentiate beneath acceptable cues. Cell adhesion is the initiating occasion on this course of; with out it, cells have restricted alternatives to provide ECM and obtain differentiation indicators. Owing to graphene’s planar construction, cells can adhere extra intently to its floor, thereby facilitating differentiation into the osteogenic lineage. Experimental outcomes affirm that graphene-based nanomaterials (GBN) upregulate the expression of a number of genes associated to adhesion and ECM manufacturing in mesenchymal stem cells (MSCs) from varied sources, thereby selling osteoblast adhesion and triggering differentiation indicators. Because of this, MSCs from varied sources exhibit related osteogenic tendencies when cultured on graphene surfaces. Liu et al. [74] efficiently synthesized nanographene oxide by way of using electrochemical derivatization, which enhanced the formation of endothelial tip cells by recruiting endogenous lysophosphatidic acid and indicated environment friendly angiogenesis in in vitro and in vivo experiments.
It’s essential to do not forget that GBN exerts a variety of complicated impacts on stem cell growth. The varied types and traits create a really completely different extracellular setting, thereby regulating stem cell conduct [75, 76]. When graphene is employed as a organic scaffold for culturing human mesenchymal stem cells (hMSCs), it demonstrates a powerful skill to advertise osteogenic differentiation whereas markedly inhibiting adipogenic differentiation. This phenomenon might be attributed to the denaturation of insulin—a key regulator of fatty acid synthesis—upon interplay with graphene, which ends from robust π-π stacking interactions. In distinction, when hMSCs are cultured on hydrophilic graphene oxide (GO), their potential for adipogenic differentiation is enhanced [77]. This discrepancy could also be as a result of presence of oxygen-containing purposeful teams on GO, which disrupt the robust π-π bonding capabilities and the in depth sp2-conjugated construction inherent to graphene. Consequently, insulin adsorbed onto GO doesn’t endure denaturation. Moreover, the excessive affinity between GO and insulin additional promotes the adipogenic differentiation of hMSCs [78]. Collectively, these findings point out that the distinct morphological and physicochemical properties of graphene-based nanomaterials (GBNs) play a pivotal position in directing the differentiation of hMSCs into particular tissue lineages.
Moreover, graphene is continuously utilized as a floor coating together with different conventional substances to enhance degrading behaviors and improve organic features [79]. Zhou et al. [80] efficiently loaded rGO on the floor of a graded porous hydroxyapatite (HA) scaffold. In vitro experiments confirmed that the degradation price of the HA/rGO composite framework matched nicely with the speed of recent bone formation and profoundly elevated the proliferation and spontaneous osteogenic differentiation of bone marrow MSCs (BMSCs) (Fig. 1B). Likewise, Kang et al. [81] proved that introducing rGO to the floor of typical titanium (Ti) scaffolds tremendously decreased the floor roughness of the Ti matrix and boosted the osteogenic differentiation of BMSCs. As a part of composite supplies, GBN can strengthen the interfacial contact between energetic molecules and conventional supplies, selling their biocompatibility [82,83,84]. As well as, varied constructions and chemical compositions could set off distinct osteogenic differentiation pathways corresponding to bone morphogenetic proteins (BMPs), reworking development factor-β (TGF-β) and Wingless-Kind MMTV Integration Website Household (Wnt) signaling pathways to realize sturdy osteogenic results within the absence of exogenous osteoinductive development components (Fig. 1C) [85].
Clinically, surgical resection is continuously required to deal with bone tumors, nonetheless, this process causes irreversible tissue injury, which has a considerable damaging affect on sufferers’ high quality of life [86]. Standard chemotherapy and radiation are neither selective nor particular in destroying cells and may result in main dangerous unintended effects, so it’s essential to analysis extra exact tumor-targeted therapeutic method [87]. PTT makes use of warmth produced by near-infrared (NIR) radiation to induce hyperthermia in tumor tissue, resulting in protein denaturation, cell membrane rupture and subsequent loss of life of most cancers cells [88]. PDT kills most cancers cells by producing singlet oxygen (SO) or reactive oxygen species (ROS) beneath gentle utilizing photosensitizer molecules [89]. 2D nanomaterials with robust NIR absorption maintain appreciable promise for PTT/PDT of bone tumors, as demonstrated by quite a few in vivo animal experiments that achieved optimistic therapeutic outcomes. Zeng et al. [19] successfully created (4-carboxybutyl) triphenyl phosphonium bromide (TPP)-conjugated indocyanine inexperienced (ICG)-loaded polyethylenimine-modified PEGylated nanographene oxide (PPG) (TPP-PPG@ICG), which may goal mitochondria and set off PTT and PDT throughout NIR irradiation, exerting a robust killing affect on drug-resistant osteosarcoma cells each in vitro and in vivo (Fig. 1D). PTT based mostly on 2D nanomaterials will also be utilized in treating bone metastases [90]. Ge et al. [91] used freeze-drying to polymerize GO nanoparticles, hydrated CePO4 nanorods and bioactive chitosan (CS) into CePO4/CS/GO porous scaffold that assist bone regeneration and vascular growth. Porous scaffolds had been utilized for the postoperative therapy of bone metastasis induced by breast most cancers. GO nanosheets successfully enhanced the photothermal conversion effectivity of photothermal remedy (PTT) in eradicating postoperative residual tumor cells, whereas CePO4 nanorods promoted angiogenesis and osteogenic exercise to facilitate bone defect restore. The scaffold was capable of elevate the native temperature to 52 °C in mice inside 30 s of near-infrared (NIR) irradiation. After two weeks of therapy, the tumor measurement within the handled mice was considerably smaller than that within the management group. Along with act as a photothermal nanotherapeutic agent, GBN concurrently transport anti-cancer drugs to tumor websites for multi-pathway anti-cancer therapy (Fig. 1E) [92]. Zhang et al. constructed polyetheretherketone (PEEK)/graphene composite scaffolds utilizing 3D printing and subsequently electrochemically deposited a hydroxyapatite-based, drug-loaded bioactive coating onto them. Graphene oxide (GO) loaded with the chemotherapeutic agent cisplatin served as an efficient photothermal (PT) converter, and the scaffold was employed for multimodal most cancers ablation remedy [92]. GO functioned as a PT converter inside the scaffold, reaching a temperature of 45 °C inside 100 s. At this elevated temperature, drug diffusion is enhanced, and near-infrared (NIR) irradiation additional accelerates the discharge of the loaded medicine. In a mouse osteosarcoma mannequin, implantation of the scaffold adopted by NIR irradiation elevated the native temperature above 45 °C inside 100 s, sustaining roughly 50 °C all through the rest of the therapy interval. After 10 days of therapy, the tumor weight was decreased by 98.5% in comparison with the management group. GO concurrently features as each a provider for chemotherapeutic medicine and a photothermal agent, exerting a synergistic impact inside this therapy platform by means of photothermal remedy (PTT)-mediated thermal injury to tumor cells and enhanced launch of anticancer medicine at elevated temperatures, thereby enhancing its antitumor efficacy. Furthermore, NIR laser induction can decrease the dosage of chemotherapy medicine and increase the effectiveness of anti-cancer remedy [93].
To summarize, GBN displays exceptional potential within the area of BTE as a result of to its distinctive honeycomb nanostructure, robust biocompatibility, coordinated biodegradability and mechanical stability in addition to nice cell-scaffold interplay capabilities. Within the meantime, the sunshine absorption traits and photothermal conversion effectivity introduced by the distinctive digital configuration along with focused remedy convey hope to the administration of bone tumors. The in depth chemical composition and various organic results make GBN a horny candidate for orthopedic medical purposes.
(A) Schematic illustration of assorted molecular constructions of GBN. Reproduced with permission from Ref [63]. Copyright 2021, John Wiley and Sons. (B) Schematic diagram of the formation means of the porous HA/rGO scaffold. After the introduction of GO in porous HA ceramics, giant GO sheets are hooked up to the floor of the through-hole construction, and small GO sheets are embedded within the gap wall. Reproduced with permission from Ref [80]. Copyright 2019, American Chemical Society. (C) The reasonable binding affinity of BMPs to GO and SiGO nanosheets allows the dynamic deposition-release of endogenous BMPs produced by cells, making a optimistic “suggestions loop”. Reproduced with permission from Ref [85]. Copyright 2021, Elsevier. (D) Mitochondria-targeted TPP-PPG@ICG nanocomposites synergistically improve antitumor efficacy after laser irradiation. Reprinted with permission from Ref [19]. Copyright 2021, Zeng et al. (E) PEEK/graphene nanocomposites loaded with antibiotics and anticancer medicine allow them to eradicate drug-resistant micro organism and ablate osteosarcoma most cancers cells, and their therapeutic results might be additional enhanced by on-demand laser-induced heating. Reprinted with permission from Ref [92]. Copyright 2021, American Chemical Society. GBN, graphene-based nanomaterials; HA, hydroxyapatite; rGO, decreased graphene oxide; GO, graphene oxide; BMPs, bone morphogenetic proteins; TPP-PPG@ICG, (4-carboxybutyl) triphenyl phosphonium bromide-conjugated indocyanine green-loaded polyethylenimine-modified PEGylated nanographene oxide; PEEK, polyether ether ketone
BP
BP, also referred to as phosphoene, is a novel type of 2D nano-layered semiconductor consisting of phosphorus atoms organized in a honeycomb construction resembling graphene. In distinction to the planar construction of graphene, BP is an orthogonal rhombic construction with corrugates, fashioned by the covalent bonding of 1 phosphorus atom with three adjoining phosphorus atoms [94]. This crystal configuration permits lamellar BP to trigger disruptions to cell membranes and show size-dependence [95]. Xing et al. synthesized black phosphorus (BP) nanosheets of various sizes for analysis. The typical thicknesses of BP-1, BP-2, and BP-3 had been 91.9 ± 32.0 nm, 27.0 ± 12.0 nm, and 17.0 ± 4.0 nm, respectively, whereas their lateral dimensions had been 884.0 ± 102.2 nm, 425.5 ± 78.8 nm, and 208.5 ± 46.9 nm, respectively [95]. When co-cultured with cells in vitro, the smallest BP-3 confirmed the bottom degree of cytotoxicity. Even on the highest focus (200 µg/mL), BP-3 exhibited solely reasonable toxicity. The cytotoxicity of BP additionally diversified amongst completely different cell sorts; for BP-1, the IC₅₀ worth in probably the most tolerant HCoEpiC cells was over 30 instances larger than that in probably the most delicate 293 T cells. At publicity concentrations under 1 µg/mL, each BP-2 and BP-3 promoted cell proliferation to some extent, presumably as a result of degradation of BP producing phosphates that stimulate cell development. BP may straight injury the cell membrane, ensuing within the launch of intracellular contents and subsequent cell loss of life. The scale of BP could affect its mode of interplay with the cell membrane; bigger nanosheets could disrupt the integrity of the lipid bilayer, inflicting leakage of intracellular contents, whereas smaller nanosheets could deposit on the membrane floor, resulting in minor disturbances. BP demonstrates important anisotropy, with lots of its properties depending on the variety of stacked layers supported by van der Waals forces [96].
Among the many most steady allotropes of phosphorus, bulk BP was first synthesized over a century in the past, whereas BP skinny movies weren’t found till 2014 [10]. Composed solely of phosphorus, a pure necessary constituent of human bone, BP-based biomaterials provide notable benefits in bone-related therapies [97]. BP displays wonderful biocompatibility, as its constituent components readily react with water and oxygen to degrade into non-toxic compounds corresponding to phosphates and phosphites, that are simply excreted by way of the urinary system [98]. Nevertheless, this chemical reactivity, coupled with a scarcity of mature artificial methods, has slowed the event of BP nanosheets in bone illnesses regardless of its promising prospects. To realize widespread utility, the steadiness of BP might be enhanced by means of encapsulation, chemical modification, and doping with different components [99]. General, BP-based biomaterials are primarily utilized in BTE and bone tumor remedy [100].
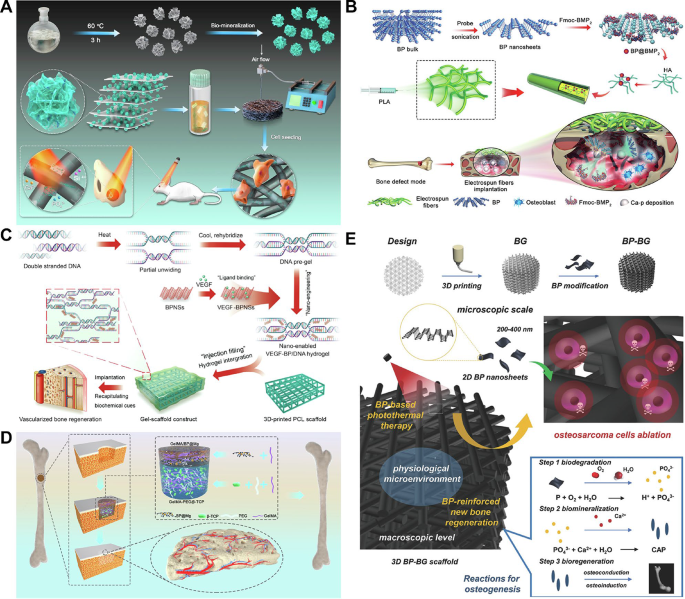
In BTE geared toward repairing giant bone defects, BP demonstrates sturdy osteogenic efficiency by means of a phosphorus-driven calcium seize course of. Upon oxidation, BP degrades into phosphates that bind with surrounding free calcium ions to kind calcium phosphate (CaP) crystal mineral deposits, thereby selling in situ bone regeneration and restore of load-bearing tissues. Enough calcium supplementation is useful for reaching an acceptable Ca/P ratio (Fig. 2A) [101]. Wang et al. [102] efficiently synthesized double community and nano-engineered (NE) hydrogels, reaching adjustable mechanical options and a biomimetic extracellular matrix microenvironment. By incorporating multifunctional 2D BP nanosheets, NE hydrogels not solely exhibit wonderful mechanical properties but in addition induce CaP mineralization beneath mildly alkaline circumstances, thus selling bone tissue regeneration.
Moreover, BP can leverage the inherent benefits of 2D nanomaterials by loading osteogenic or angiogenic medicine to realize corresponding functionalities. BP nanosheets modified with bone morphogenetic protein-2 (BMP-2) facilitate the recruitment of osteoprogenitor cells and promote their differentiation into mature osteoblasts, thereby initiating the bone regeneration course of (Fig. 2B) [103]. Liu et al. [104] constructed 2D heteronano-layers utilizing BP and GO as the premise for purposeful spheroid design, geared toward offering a cell-guiding microenvironment for big bone defect restore. GO nanosheets improve cell adhesion, migration, proliferation, and differentiation, whereas additionally enhancing the mechanical properties of biomaterials. In distinction, BP nanosheets possess an necessary attribute for orthopedic purposes: their degradation merchandise are phosphates, which aren’t solely innocent to the human physique but in addition function important ions for orthopedic and skeletal growth, taking part in an important position in osteogenesis and bone integration. The mixture of those two nanosheets can complement one another’s benefits. On this composite materials, bone marrow mesenchymal stem cell spheroids are used to straight ship stem cells to the defect web site, decreasing the time required for cell recruitment and accelerating the regeneration course of. In each in vitro and in vivo experiments, these engineered spheroids had been proven to advertise stem cell proliferation and osteogenic differentiation, demonstrating their osteogenic assist in a rat calvarial defect mannequin. Moreover, by loading the osteogenic inducer dexamethasone, these scaffold-free spheroids achieved enhanced osteogenic induction with out prior cultivation in osteogenic medium. Angiogenesis gives important vitamins and oxygen, selling the expansion and restore of recent bone tissue. The involvement of the nervous system contributes to angiogenesis and osteogenesis by releasing neuropeptides and different signaling molecules, thus taking part in an important position in bone regeneration. In Miao et al.’s examine, BP nanosheets loaded with vascular endothelial development issue (VEGF) enabled dynamic DNA hydrogels to be mixed with 3D-printed scaffolds, developing a bioactive gel scaffold construction. VEGF non-covalently certain with BP maintained sustained launch, stimulating the migration of human umbilical vein endothelial cells (HUVECs), thereby offering enough vascularization for bone defect restore (Fig. 2C) [105]. Xu et al.’s analysis demonstrated an progressive neurovascularized bone regeneration technique by means of the design of a bilayer hydrogel. The higher layer of Mg-coordinated BP hydrogel mimicked the construction of the periosteum, considerably selling angiogenesis by enhancing endothelial cell migration and neural-related protein expression in neural stem cells. The decrease hydrogel layer successfully enhanced the exercise and osteogenic differentiation of BMSCs. This examine additionally validated the bone regeneration impact of the bilayer hydrogel scaffold in a rat calvarial defect mannequin, indicating that this technique provides a novel method for designing neurovascular community biomaterials for bone regeneration (Fig. 2D) [106]. Though the degradation merchandise of BP are non-toxic and promote osteogenic differentiation, BP nanosheets exhibit poor stability beneath aqueous and oxygen-rich circumstances. Oxygen molecules on the floor of BP generate superoxide ions, which react with the lone-pair electrons on BP to kind oxidized phosphorus. Within the presence of water, the oxidized phosphorus quickly degrades into phosphates. If degradation happens through the preliminary implantation section and even previous to implantation, it could negatively affect the ultimate therapeutic final result. Subsequently, enhancing the steadiness of BP is important. Combining BP with different supplies might help deal with the problem of speedy degradation. Moreover, such combos can impart multifunctional properties to the composite materials, enhancing bioactivity whereas additionally offering extra purposeful traits. In orthopedic purposes, BP additionally faces limitations in mechanical properties, as supplies composed solely of BP can not present ample mechanical assist for bone regeneration. Subsequently, within the therapy of orthopedic illnesses, BP is commonly mixed with scaffold supplies. The first operate of scaffold supplies is to offer mechanical assist, guaranteeing that the mechanical properties of the composite align with these of bone tissue to stop untimely collapse and stress shielding. Scaffold supplies can even incorporate different elements, integrating a number of complicated properties to dynamically regulate bone regeneration in each time and area. Combining BP with scaffolds is an indispensable technique for advancing the scientific translation of BP-based supplies [98].
As a direct-bandgap, metal-free semiconductor, BP displays exceptionally excessive effectivity and efficiency in photoelectric conversion. Furthermore, the bandgap of BP might be tuned in line with the variety of layers (starting from 0.3 to 2.0 eV), facilitating broad-spectrum gentle absorption from seen to mid-infrared, thereby filling the bandgap void on this vary left by graphene and TMDs [107]. Tong et al. [108] demonstrated that beneath NIR irradiation, BP displays an environment friendly photothermal response, considerably heating even when lined by organic tissues as much as 7 mm thick. The photothermal properties of BP current distinct benefits in treating infectious bone defects. Jing et al. [109] proposed a novel technique for infectious bone defects by using a photosensitive conductive hydrogel for therapy. This hydrogel combines the antibacterial properties of magnesium-modified black phosphorus with photothermal/photodynamic therapeutic results, successfully enhancing the infection-induced microenvironment and selling the reconstruction of neural networks and bone regeneration. The examine outcomes indicated that this hydrogel exhibited wonderful antibacterial efficiency beneath NIR irradiation, decreasing the inflammatory response. This progressive technique provides new instructions for treating infectious bone defects, emphasizing the essential position of nerves in bone restore, and gives potential new avenues for future therapies. Impressed by pure chloroplasts, Zhao et al. [110] developed a scaffold with photothermal stability and antibacterial properties. By inducing a multifunctional coating of polydopamine, BP was efficiently stabilized within the scaffold, with the simultaneous development of silver nanoparticles. This scaffold not solely captures NIR gentle and converts it into thermal vitality but in addition achieves pH-responsive launch of silver ions and enhanced photothermal stability.
Practically all kinds of bone defects endure an inflammatory response as an preliminary stage, encompassing an early acute pro-inflammatory response adopted by later irritation decision. This course of is primarily utilized for cell recruitment, clearance of necrotic tissue, secretion of cytokines and development components, and promotion of cell differentiation. Macrophages, as the most typical effector immune cells through the inflammatory section, activate osteogenic signaling pathways and take part in all levels of bone therapeutic [111]. Qiu et al. [112] revealed the significance of accelerating bone regeneration by modulating the inflammatory course of and proposed a novel BP-based therapeutic technique. The examine demonstrated that BP may improve the inflammatory response within the early stage and promote irritation decision within the subsequent stage, thus creating an immune microenvironment conducive to bone regeneration. Moreover, BP straight promotes osteogenic differentiation of BMSCs by stimulating macrophage-mediated interleukin-33 (IL-33) expression, additional facilitating bone restore. This examine gives new proof for utilizing BP to modulate immune responses to speed up bone regeneration and provides potential methods for future scientific therapies. Wu et al. [113] found that multifunctional hydrogels loaded with BP nanosheets exhibit wonderful NIR/pH dual-responsive properties, which not solely promote osteogenesis and angiogenesis but in addition display the power to scavenge extreme ROS and induce macrophage polarization in the direction of the M2 phenotype. Furthermore, this hydrogel platform can drive the secretion of purposeful cytokines, accelerating bone regeneration.
Given their excessive biocompatibility, biodegradability, photothermal effectivity and drug-loading capability, BP nanosheets have additionally been extensively studied for bone tumor remedy and anti-infection engineering (Fig. 2E) [114,115,116,117,118]. Zhao et al. [119] efficiently developed a biomineralization-inspired CS/hydroxypropyltrimethyl ammonium chloride chitosan/HA/BP (CS/HC/HA/BP) hybrid scaffold for treating extreme problems related to bone tumors, corresponding to tumor recurrence, an infection and in depth bone loss. This scaffold possesses a bone-like hierarchical porous construction, enhancing bioactivity, osteoconductivity, and mechanical properties by regulating the HC/CS community matrix and in-situ crystallized HA nanoparticles distribution. The hybrid scaffold stabilized by HC displays good photothermal conversion properties, with the conversion temperature adjustable by way of BP focus and NIR energy density. In vitro and in vivo research demonstrated the efficacy of a one-step NIR-mediated protection remedy, together with antibacterial and antitumor results, at roughly 49 °C. Subsequently, at round 42 °C, the scaffold promotes osteogenesis by upregulating the expression of osteogenesis-related genes. Wu et al. [120] ready a promising antibacterial nanomedicine (ZnL2-BPs@HAP) by incorporating BP into the floor of 3D-printed HA scaffolds by way of ZnL2 coordination. ZnL2-BP not solely imparts photothermal properties to the HA scaffold but in addition renders contacted micro organism extra delicate to excessive temperatures. Below circumstances decrease than typical PTT temperatures, ZnL2-BPs@HAP scaffolds exhibited wonderful antibacterial photothermal effectivity with minimal facet impact. Moreover, in one other examine, BP nanosheets (BPNS) successfully protected chondrocytes from ROS-induced degradation as a ROS scavenger and promoted osteogenic differentiation of BMSCs by means of its biomineralization functionality, thereby reaching subchondral bone restore. In a rat OA mannequin, BPNS confirmed effectiveness in preserving cartilage morphology and subchondral bone quantity, making it a promising nanoplatform for early OA therapy [121].
In abstract, BP-based nanomaterials signify a extremely promising orthopedic implant materials, addressing most cancers therapy, antibacterial motion and osteogenesis concurrently. Their distinctive organic responses provide important growth potential within the area of bone remedy, and their mixture with 3D printing considerably mitigates the shortcomings in fabrication strategies. BP-based nanomaterials are step by step changing into a perfect alternative for orthopedic medical purposes.
(A) Illustrating the fabrication of 3D BP@HA. BP nanosheets exert wonderful photothermal properties and in addition present a P supply for bone regeneration by means of biomineralization. Reprinted with permission from Ref [101]. Copyright 2021, Li et al. (B) Schematic illustration of the method of fabrication of BP@BMP-2 electrospun fiber scaffolds, in addition to in vivo osteoblast recruitment and phosphate mineralization results. Reprinted with permission from Ref [103]. Copyright 2020, John Wiley and Sons. (C) Schematic idea of bioactive gels for VEGF-modified BPNSs to boost vascularized bone regeneration. Reprinted with permission from Ref [105]. Copyright 2022, Miao et al. (D) Diagrammatic illustration of a bilayer hydrogel scaffold that concurrently induces neurovascular regeneration and osteogenesis. Reprinted with permission from Ref [106]. Copyright 2022, Xu et al. (E) Schematic diagram of a 3D printed BP-BG scaffold for photothermal remedy and subsequent bone regeneration for osteosarcoma. Reprinted with permission from Ref [114]. Copyright 2018, John Wiley and Sons. 3D, three dimensions; BP, black phosphorus; BMP-2, bone morphogenetic protein-2; VEGF, vascular endothelial development issue; BPNP, BP nanosheets; BG, bioactive glass
Nanoclays
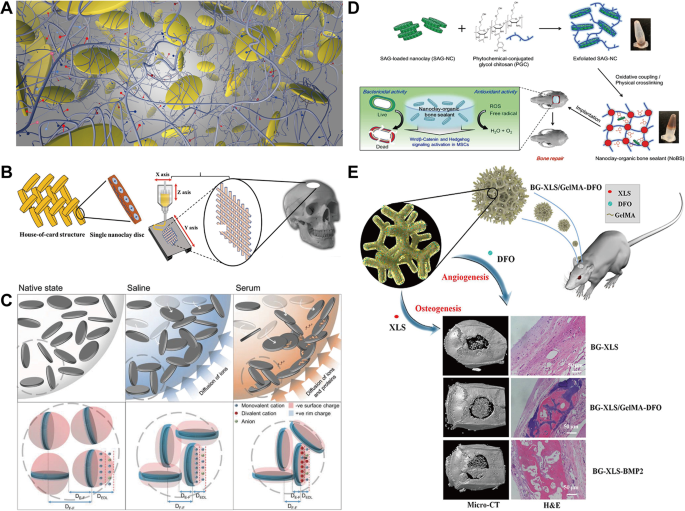
Nanoclays are synthetic or natural layered silicate particles composed of tetrahedral sheets (SiO4) linked with octahedral sheets [11]. Primarily based on their chemical composition and nanoparticle morphology, nanoclays might be categorized into a number of sorts, corresponding to smectite (montmorillonite, saponite), kaolinite, pyrophillite and halloysite nanotube [15]. Laponite is a consultant with method expressed as Na+ 0.7 [Si8 Mg5.5 Li0.3O20(OH)4]−0.7, which has a everlasting damaging cost dispersed throughout the floor of the particle coupled with a optimistic cost alongside the sides [122]. The disc-shape morphology of nanoclays gives a excessive particular floor space for the attachment of biomolecules, and the twin expenses distribution facilitates interactions on the edge websites of those biomolecules. One examine has proven that bisphosphonates functionalized on the edge websites of laponite assist keep the native focus of energetic proteins in vivo, thereby enabling bone induction at considerably decrease doses of BMP-2 than normally required [123].
Nanoclays exhibit excessive biocompatibility, primarily as a result of most of them include minerals naturally current within the physique and degrade beneath physiological circumstances into non-toxic merchandise that may induce osteogenic differentiation. As an illustration, Mg2+ and Li+ take part in activating osteogenic regulatory pathways and selling new bone formation, whereas Si and Si(OH)4 play important roles in angiogenesis and the calcification of bone tissue through the regeneration course of [124,125,126]. Along with direct contact, nanoclay-based scaffolds are broadly utilized in bone and cartilage tissue engineering [127]. Firstly, the incorporation of nanoclays enhances the mechanical energy and degradation properties of composite scaffolds, yielding porous constructions with mechanical attribute much like native bone matrices (Fig. 3A, B) [128, 129]. Hu et al. [130] considerably strengthened the mechanical and organic efficiency of methacrylated gelatin (GelMA) hydrogels by incorporating laponite nanoclays, making a steady microenvironment that helps cell proliferation and differentiation. Furthermore, the nanoclays permitted the managed launch qualities of hydrogel, permitting human umbilical cord-derived mesenchymal stem cell-derived small extracellular vesicles (hUC-MSCs-sEVs) to be step by step launched because the hydrogel degraded, which in flip inspired cartilage regeneration by way of activation of the PTEN/AKT signaling pathway. In vivo experiments confirmed the effectiveness of this GelMA/nanoclay/sEV hydrogel in repairing cartilage defects. The incorporation of nanoclay into the hydrogel considerably enhanced its resistance to degradation. After 14 days, the hydrogel was fully degraded, whereas the nanoclay-reinforced hydrogel exhibited solely ~ 20% degradation and demonstrated enhanced mechanical properties. In an identical examine, Yin et al. [131] launched glycine-modified attapulgite (GATP), a pure nanometer-scale magnesium silicate mineral, into poly(1,8-octanediol-co-citrate) (POC) scaffolds, facilitating their compressive energy and biocompatibility whereas making a conducive microenvironment for each cartilage and subchondral bone regeneration. The inclusion of GATP neutralized the acidity of citrate, decreased early inflammatory responses, and considerably promoted osteochondral differentiation. In a rabbit articular cartilage defect mannequin, the POC/GATP composite scaffolds demonstrated wonderful ECM secretion and osteochondral regeneration capabilities. Secondly, nanoclays act as bioactive components that may promote mobile features, encouraging cell adhesion, proliferation, and differentiation, generally reaching these outcomes with out the necessity for extra development issue or stem cell supply (Fig. 3C) [132, 133]. The degradation behaviour of the POC/GATP composite scaffold in phosphate-buffered saline (PBS) resolution is taken into account excellent, exhibiting a mass lack of roughly 22.48% after an 8-week immersion interval. Moreover, it displays a compressive modulus corresponding to that of articular cartilage. In vivo experiments demonstrated that the POC/GATP scaffold exhibited restore results corresponding to these of pure cartilage. Nevertheless, these organic processes are at present supported solely by gene-level knowledge, and investigations into the long-term stability and degradation behaviour in vivo stay incomplete. Future analysis ought to prioritize these efficiency points. Lee et al. [134] developed a nanoclay-organic hydrogel bone sealant (NoBS) by co-assembling inorganic silica-rich nanoclays with phytochemical-modified CS to imitate the extracellular microenvironment (ECM) of bone tissue. On this system, nanoclays not solely enabled the osteoconductivity and bodily traits of the hydrogel, but in addition promoted osteoinduction by regulating the Wnt/β-catenin signaling pathway. With the added advantages of antibacterial, antioxidant and self-healing options, NoBS has emerged as a promising various for bone defect restore, demonstrating appreciable scientific potential (Fig. 3D). Thirdly, the presence of nanoclays can foster cell-material interactions by means of floor modification and functionalization, creating a good microenvironment for osteoblast conduct. Excessive-throughput transcriptome sequencing has revealed that after mobile endocytosis, nanoclays activate stress response pathways, stimulating the differentiation of hMSCs in the direction of osteogenic and chondrogenic lineages [135]. Zheng et al. [136] fabricated a laponite-functionalized 3D bioactive glass (BG) scaffold, the place the incorporation of laponite considerably boosted the mechanical properties of the scaffold and improved osteogenic differentiation of adipose-derived stem cells (ADSCs). Below simulated hypoxic setting, the expression of hypoxia-inducible factor-1α (HIF-1α) and VEGF was upregulated, leading to enhanced angiogenesis and bone regeneration each in vitro and in vivo (Fig. 3E). Regardless of these benefits, nanoclay nonetheless faces limitations concerning its long-term stability and degradation behaviour in vivo. The first problem lies within the mismatch between its degradation price and the speed of bone tissue regeneration. This temporal and spatial mismatch not solely weakens the mechanical assist operate of the scaffold however may trigger collapse on the bone defect web site, finally compromising the efficacy of bone regeneration remedy. Resulting from complicated and multifaceted degradation mechanisms, nanoclay struggles to synchronize its degradation with different supplies, doubtlessly leading to untimely scaffold collapse or localized stress focus, thereby resulting in failed bone restore. Moreover, degradation merchandise of nanoclay could trigger long-term biocompatibility issues, as nano-sized silicate fragments produced throughout degradation can accumulate in organs such because the liver and spleen, thus affecting biosafety. The weakly alkaline microenvironment that will kind domestically throughout degradation may additionally have an effect on osteoclast exercise, interfering with mineralization and osteogenesis of the extracellular matrix. Limitations in manufacturing processes could additional affect the degradation behaviour of nanoclay supplies in vivo. Inadequate dispersion uniformity throughout manufacturing can result in native aggregation of nanoclay particles, disrupting uniform degradation and finally leading to unstable mechanical properties or scaffold collapse.
Via cation alternate, electrostatic adsorption, hydrogen bonding and cation bridging, nanoclays may readily bind to biomolecules corresponding to proteins, offering a sustained launch of assorted necessary development components and medicines to the mobile microenvironment [137]. Resulting from their flat, negatively charged sides, layer-by-layer movies are fashioned by way of the parallel adsorption of flake laponite onto positively charged surfaces. Moreover, the disc-like morphology of laponite impedes macromolecular diffusion, making a tortuous path that considerably slows the discharge price of loaded molecules between the layers. Primarily based on this construction, Howard et al. used low-dose BMP-2 to considerably kind well-structured Haversian methods in a rat calvarial defect mannequin [138].
As a novel class of biomaterials, nanoclays possess distinctive layered morphology and cost distribution, permitting them to work together with scaffold supplies, cells and ECM elements. Nanoclays are simply synthesized and cost-effective, with controllable sizes and outlined constructions when used as precursor supplies for 3D bioprinting, responding to bone induction signaling pathways [139]. Nanoclay interacts with cells and biomolecules inside the osteogenic microenvironment to exert osteogenic results. Magnesium ions (Mg2+) and silicon ions (Si4+) launched from nanoclay activate osteogenic-related signalling pathways, together with BMP/Smad and Wnt/β-catenin, which promote the activation of osteogenic-specific transcription components corresponding to Runx2, Osterix, and osteopontin, thereby inducing the differentiation of bone marrow mesenchymal stem cells (BMSCs) into osteoblasts [140]. Silicon ions additionally promote angiogenesis coupled with osteogenesis by stabilizing hypoxia-inducible issue 1α (HIF-1α), thereby activating the VEGF axis and offering dietary assist for bone regeneration [141]. The negatively charged surfaces of nanoclay can adsorb calcium and phosphate ions by means of electrostatic interactions, thereby forming mineralization precursors and accelerating bone matrix mineral deposition. Moreover, montmorillonite inhibits osteoclast differentiation and bone resorption by downregulating the expression of key genes, together with NFATc1 and TRAP [142].
Nanoclays can even bind biomolecules by way of electrostatic interactions, enabling managed launch of development components. For instance, the negatively charged layers of nanoclays can bind to the positively charged amino acid domains of bone morphogenetic protein-2 (BMP-2), whereas their hydrated floor layers assist keep BMP-2’s native conformation, thereby preserving its bioactivity and enabling sustained launch. Within the complicated bone microenvironment, the concentrating on specificity of nanoclay might be enhanced by way of floor modification. Conjugation of concentrating on ligands, corresponding to bisphosphonates or RGD peptides, allows nanoclay to particularly goal bone minerals or osteoblasts. Moreover, biomimetic mineralization methods might be employed by in situ deposition of hydroxyapatite on the nanoclay floor to imitate pure bone tissue, thereby enhancing bone conductivity. Managed drug launch from nanoclay carriers can make the most of a number of response mechanisms, together with protonation of nanoclay in acidic environments to set off drug launch, or enzyme-responsive methods concentrating on particular enzymes within the bone microenvironment, corresponding to matrix metalloproteinases (MMPs) [143, 144].
In designing bone-based composite supplies utilizing nanoclay, optimizing the focus and spatial distribution of nanoclay is important to reaching a stability between mechanical properties and organic exercise. The addition of nanoclay imparts bioactivity to the composite materials. At low concentrations, nanoclay might be uniformly dispersed inside the matrix, enhancing the compressive modulus of the scaffold by way of non-covalent interactions, together with hydrogen bonding and electrostatic forces, whereas sustaining excessive porosity to facilitate nutrient transport and cell migration. Nevertheless, extreme nanoclay can result in stress focus and heterogeneous degradation charges as a result of agglomeration, thereby impairing osteogenic efficiency. For instance, Yin et al. [131] reported that the addition of GATP successfully elevated the compressive modulus of POC scaffolds, with a ten% addition approaching that of articular cartilage. Nevertheless, when the GATP focus elevated to fifteen%, the compressive modulus of the scaffold decreased, as extreme GATP crammed small pores, decreasing inside connectivity and leading to uneven stress distribution inside the scaffold beneath compression. Methods corresponding to floor modification or dynamic cross-linking community design can mitigate limitations related to nanoclay focus. Relating to the spatial distribution of nanoclay, it should align with the gradient construction of bone-cartilage tissue, characterised by decrease mineralization within the cartilage layer and better mineralization within the bone layer. Programmable management of nanoclay density might be achieved by means of strategies corresponding to gravity permeation, 3D printing, hot-press curing, and multi-material co-assembly to fulfill the mechanical load necessities of assorted anatomical areas. The important thing to regulating nanoclay focus and distribution lies in real-time synchronous monitoring of parameter modifications and optimization of producing processes, which can be important for scalable nanoclay manufacturing [145].
Future efforts ought to prioritize the event of clinically related animal fashions, set up manufacturing standardization (together with GMP-compliant manufacturing processes), and conduct preclinical research that adjust to regulatory necessities to complement in vivo knowledge on the long-term biosafety, systemic clearance, and inflammatory responses of nanoclay, in addition to its interactions with complicated human tissues, significantly beneath pathological or immunocompromised circumstances. Moreover, efforts ought to deal with integrating nanoclay into bio-manufacturing platforms, corresponding to 3D bioprinting and injectable hydrogels, to boost spatiotemporal management over drug supply and tissue formation.
(A) Schematic drawing of the formation of a composite hydrogel by covalent crosslinking of nanosilicate and GelMA beneath ultraviolet irradiation. Reprinted with permission from Ref [128]. Copyright 2015, American Chemical Society. (B) Laponite with negatively charged surfaces and positively charged edges can kind a “home of playing cards” construction by means of edge-to-edge interactions. Laponite utilizing 3D bioprinting improves the mechanical energy of bone implants. Reprinted with permission from Ref [129]. Copyright 2019, John Wiley and Sons. (C) Diagrammatic presentation of the mechanism by which Laponite diffusion gels are fashioned in numerous liquid environments. Reprinted with permission from Ref [132]. Copyright 2018, John Wiley and Sons. (D) Schematic diagram of the co-assembly of inorganic nanoclay and natural phytochemically enhanced chitosan to realize self-healing, antibacterial and antioxidant actions. Reprinted with permission from Ref [134]. Copyright 2020, John Wiley and Sons. (E) Laponite-functionalized BG scaffold with hypoxic properties that promotes angiogenesis and osteogenesis. Reprinted with permission from Ref [136]. Copyright 2021, Zheng et al. GelMA, methacrylated gelatin
LDHs
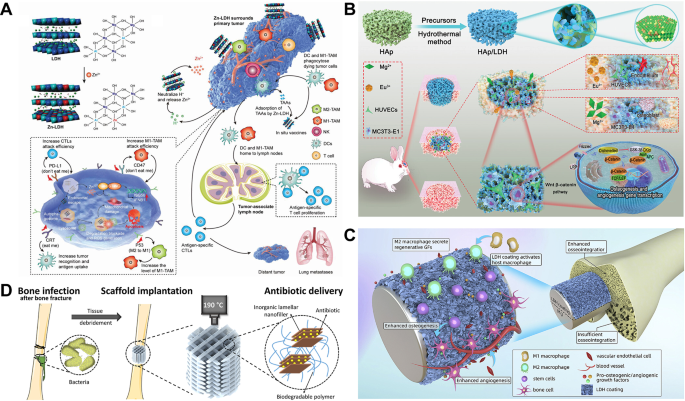
LDHs, also referred to as anionic clays, are compounds composed of positively charged layers and interlayer anions stacked collectively by way of electrostatic interactions. Their normal method is [M1−x2+Mx3+(OH)2]x+(An−)x/n·mH2O, the place M2+ and M3+ signify divalent and trivalent steel cations respectively and An− refers to exchangeable interlayer anions [23]. The steel ions inside the layers of LDHs show exceptional tunability, with distinct steel ions mediating particular pathophysiological processes. As an illustration, Mg-LDHs facilitate the proliferation of osteoblasts and exhibit superior anticorrosion properties [146]. Zn-LDHs and Cu-LDHs mitigate oxidative stress by means of anti-inflammatory and antioxidant qualities, thereby enabling antimicrobial and antitumor immunity (Fig. 4A) [147,148,149]. Additionally, europium-based LDHs improve vascularization and promote the restore of each gentle and arduous tissues (Fig. 4B) [150].
LDHs have garnered in depth consideration within the area of prescription drugs supply. Their intrinsic optimistic cost encourages non-covalent interactions with anionic medicine, biomaterials and antibodies, considerably boosting transmembrane internalization and intracellular drug supply effectivity. Moreover, the presence of hydroxides imparts acid sensitivity to LDHs, rendering them excellent candidates for pH-responsive drug carriers in most cancers remedy. Drug-loaded LDHs exhibit managed launch traits, whereby intercalated medicine are step by step liberated, decreasing the frequency of administration. Lv et al. [151] developed a wise, injectable, thermosensitive hydrogel (CSP-LB) by incorporating BMP-2-functionalized MgFe-LDH nanosheets right into a chitosan/silk fibroin (CS) hydrogel loaded with platelet-derived development factor-BB (PDGF-BB). On this hydrogel system, the comparatively weak interplay between PDGF-BB and the CS hydrogel, in distinction to the robust electrostatic interplay between BMP-2 and the LDH nanosheets, enabled speedy PDGF-BB launch coupled with sustained BMP-2 launch, thus reaching environment friendly and extended bone regeneration.
Within the realm of BTE, the mixing of LDHs not solely will increase the bioactivity of inorganic matrices, selling osteoblast adhesion and proliferation, but in addition serves as a coating on metallic substrates. Such coatings successfully retard in vivo degradation, enhancing mechanical stability. For instance, Wang et al. [152] demonstrated that MgAl-LDHs successfully fostered the bioactivity and utility potential of polymethyl methacrylate (PMMA) bone cement. LDH incorporation notably decreased the polymerization temperature of PMMA, assuaging stress shielding results whereas selling sturdy bone cement interfacial integration and markedly enhancing bone formation in vivo. Equally, research revealed that MgAl-LDHs on pure magnesium surfaces efficiently induced the differentiation of osteoblasts (MC3T3-E1) and the angiogenic functionality of HUVECs. Moreover, LDH coatings induced macrophage polarization towards the anti-inflammatory M2 phenotype, optimizing the native immune microenvironment and suppressing the activation of the NF-κB signaling pathway (Fig. 4C) [153]. Zhang et al. [154] fabricated Mn-LDH nanosheet-modified magnesium-based implants, discovering that the black LDH movie, by means of its superior photothermal impact and nanozyme-like Fenton exercise, effectively eradicated osteosarcoma cells and tissues beneath NIR gentle whereas concurrently exerting photothermal antibacterial capacities. Furthermore, the LDH movie tremendously raised the corrosion resistance of magnesium-based implants, exactly managed the discharge of Mg2+ and Mn2+, and synergistically stimulated cell adhesion, proliferation and osteogenic differentiation, thereby accelerating bone regeneration. A novel examine reveals that LDH coatings might be tailor-made throughout a number of scales to match the structural and purposeful necessities of 3D-printed magnesium scaffolds, guaranteeing each biocompatibility and managed degradation. On the macroscopic scale, the scaffold displays an anatomically exact geometry, enabling seamless integration with bone defects. On the mesoscale, an optimized porous structure gives mechanical assist whereas facilitating mobile proliferation and vascularization. On the microscale, a dual-layer coating comprising a high-temperature oxidation (HTO) movie and an LDH layer establishes a protecting barrier, mitigating speedy magnesium degradation whereas modulating the microenvironment to boost osteogenic exercise. On the nanoscale, LDH nanosheets enhance floor roughness and hydrophilicity, thereby selling cell adhesion and differentiation. The synergistic interaction throughout these hierarchical ranges ensures structural integrity whereas accelerating bone tissue regeneration, providing a promising avenue for personalized bone restore options [155].
The antimicrobial options of LDHs have additionally been extensively investigated. By intercalating ciprofloxacin and gentamicin into the interlayer area of MgAl-LDHs, Cámara-Torres et al. [16] efficiently achieved sustained native antibiotic launch inside 3D-printed scaffolds to stop implant-associated bone infections. Importantly, these scaffolds supported bone tissue formation with out impairing the osteogenic differentiation or marker expression of hMSCs, demonstrating wonderful biocompatibility (Fig. 4D). As well as, Bian et al. [156] developed a BG-based composite (BGS/MCFS) functionalized with sulfide nanosheets derived from MgCuFe-LDHs, addressing prosthesis-associated infections and loosening. The antibacterial efficacy of LDHs primarily stems from their inhibition of bacterial vitality manufacturing and metabolism. Mixed with distinctive NIR photothermal conversion talents, BGS/MCFS considerably elevated temperatures beneath irradiation, enabling full eradication of micro organism surrounding implants.
In conclusion, LDH-based nanomaterials provide a extremely tunable and multifunctional platform for orthopedic purposes, able to integrating osteoinductive, angiogenic, anti-inflammatory and antimicrobial properties right into a single assemble. Via rational choice and mixture of steel ions, interlayer engineering and responsive drug loading, LDHs might be tailor-made to deal with various scientific eventualities from enhancing osseointegration to combating implant-associated infections and supporting tumor resection margins.
(A) Zn2+-doped LDH for tumor microenvironment regulation and tumor-specific immune induction. Reprinted with permission from Ref [148]. Copyright 2022, John Wiley and Sons. (B) Schematic illustration of in situ development of MAE-LDH nanosheets on porous HA scaffolds. The composite materials owns wonderful angiogenic and osteogenic properties. Reprinted with permission from Ref [150]. Copyright 2022, Wang et al. (C) MgAl-LDH coating promotes immune response of magnesium implants and displays higher bone integration efficiency. Reprinted with permission from Ref [153]. Copyright 2020, Cheng et al. (D) Antibiotic-loaded 3D printed MgAl-LDH scaffolds for bone regeneration and an infection prevention. Reprinted with permission from Ref [16]. Copyright 2020, Cámara-Torres et al. LDH, layered double hydroxides; MAE-LDH, MgAlEu-LDH
TMDs
2D TMDs, which share an identical construction to graphene, include MX₂-type crystals, the place M represents transition metals from teams IV to X (Mo, W, V and Ti) and X denotes chalcogenides (S, Se and Te). In every monolayer, TMDs characteristic a “sandwich-like” building with a layer of steel atoms sandwiched between two layers of chalcogen atoms, bonded covalently inside the layer. Vertically, adjoining layers are held collectively by weaker van der Waals forces, permitting for facile exfoliation into 2D nanosheets with out disrupting the interior bonding [157]. Platinum diselenide (PtSe2), in its bulk kind, displays an oblique bandgap, however transitions to a direct bandgap semiconductor when flaked right into a monolayer, making it extremely appropriate for purposes in photosensitive drug supply and PTT (Fig. 5A) [158, 159].
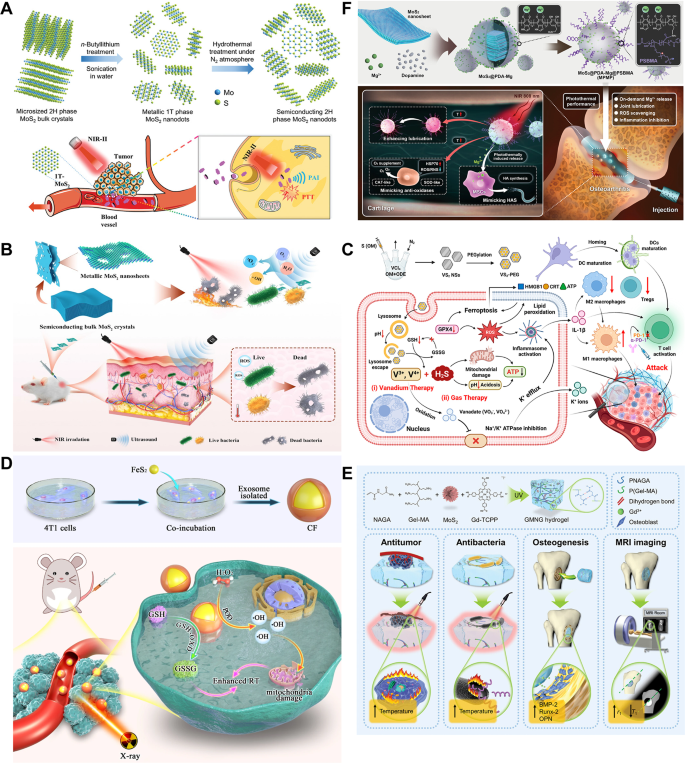
The crystal section is a important determinant of the properties and functionalities of TMDs, and section engineering permits for exact modulation of crystal section transitions, thus endowing TMDs with multifaceted chemical traits [160]. Molybdenum disulfide (MoS₂), for instance, exists in 4 distinct crystalline constructions, particularly 2H, 1 T, 1T’, and 3R, with the semiconductor 2 H section and metallic 1 T section being probably the most prevalent [161]. The two H section represents the thermodynamically steady type of MoS₂ and, when stimulated by gentle or electrons, will increase osteoblast proliferation and differentiation whereas sustaining wonderful biocompatibility. Conversely, the metallic 1 T section, obtained from 2 H by way of chemical therapies corresponding to intercalation of alkali steel lithium, displays superior conductivity, heightened catalytic exercise and enhanced antibacterial properties, rendering it advantageous for antitumor therapies (Fig. 5B) [159, 161,162,163].
By adjusting synthesis strategies and course of parameters, completely different crystalline phases of transition steel dichalcogenides (TMDs) might be obtained. Mechanical exfoliation, the earliest bodily technique, suffers from weak interlayer interactions, leading to loosely stacked layers. Ion intercalation and exfoliation use ions that react with water to generate fuel, thereby separating the layers; lithium ion intercalation, specifically, permits exact management over the important thing construction of TMDs. Moreover, liquid-phase exfoliation and chemical vapor deposition are generally employed manufacturing strategies. Optimizing these synthesis processes allows section management of TMDs [164]. Park et al. developed a melt-metal-assisted intercalation (MMI) technique leveraging the capillary motion of molten potassium and the distinction in electron affinity between MoS2 and potassium’s ionization potential. The extremely reactive molten potassium readily intercalates into MoS2 interlayers, decreasing the vitality barrier for the 1 T section transition and stabilizing the 1 T crystal construction with section purity exceeding 92%. The suppression of electron emission by Okay–S ionic bonds imparts wonderful thermal and photostability [165]. Excessive-temperature therapy of alkali steel salts in decreasing atmospheres can even drive section transitions, the place the salts act to decrease vitality limitations and supply electron doping. Moreover, colloidal thermal injection expertise permits direct development of single-layer 1T’-TMD crystals by regulating ligand supersaturation with precursors [166]. Chen et al. ready 1T/1T’-MoS2 by way of chemical lithium intercalation exfoliation by immersing 2 H-MoS2 in n-butyl lithium resolution to induce section transformation, adopted by sonication in water to acquire water-dispersible MoS2. In addition they demonstrated that easy scorching water therapy beneath nitrogen ambiance can revert 1T/1T’-MoS₂ again to the two H section.
Totally different crystalline phases of MoS2 exhibit distinct properties, making section purity important for the reproducibility and efficacy of TMDs in orthopaedic purposes. Mutalik et al. investigated the optical variations between the two H and 1 T phases of MoS2: 1T-MoS2, as a result of its metallic nature, exhibits larger absorbance at 808 nm, whereas 2 H-MoS2 displays very low absorption within the near-infrared window owing to its vast bandgap (~ 1.83 eV). Relating to photothermal efficiency, the 1 T section converts near-infrared gentle to warmth extra quickly and effectively, reaching temperatures above 70 °C inside 7 min, considerably outperforming the two H section, which solely reaches 50 °C. In antimicrobial experiments, 1T-MoS2 successfully decreased E. coli populations by inflicting in depth injury to bacterial cell partitions and disrupting development, attributed to its metallic conductivity enhancing bacterial toxicity and photothermal impact [167]. Chen et al. additional confirmed the superior photothermal impact of 1T/1T’-MoS2 in comparison with 2 H-MoS2 beneath 1064 nm laser irradiation: the 1T/1T’-MoS2 resolution reached 58.9 °C inside 3 min, whereas the two H-MoS2 resolution reached solely 35.8 °C. As a sonosensitizer, 1T/1T’-MoS2 generated roughly 45% extra reactive oxygen species (ROS) beneath ultrasonic therapy than 2 H-MoS2. In antibacterial assays, 1T/1T’-MoS2 achieved bactericidal charges of 18% and 20% towards Pseudomonas aeruginosa beneath ultrasonic and light-weight therapies respectively, with mixed therapy reaching practically 100% efficacy. These outcomes had been corroborated in vivo. Nevertheless, Chen et al. famous that the purity of 1T/1T’-MoS2 was nonetheless restricted, with round 30% of nanosheets remaining as the two H section [163].
Subsequently, section purity is important for reproducible and efficient TMD purposes in orthopaedic illness therapy. It straight impacts batch consistency and considerably influences bodily and chemical properties corresponding to floor vitality and degradation price, which in flip affect photothermal efficiency. The metallic 1 T section’s zero-bandgap construction grants it robust gentle absorption and decrease excitation vitality thresholds, enhancing ROS era upon activation, thereby making it superior for photothermal remedy. In distinction, the semiconducting 2 H section could also be higher fitted to selling osteoblast differentiation by way of managed ion launch.
Within the context of orthopedic illness administration, TMDs display huge potential throughout a number of purposes. MoS₂ nanosheets, characterised by their robust NIR absorption and excessive photothermal conversion effectivity, function excellent brokers for PTT whereas additionally regulating mobile actions [168]. Moreover, the distinctive digital construction of transition metals confers catalytic properties on TMDs, enabling them with distinctive peroxidase (POD)-mimetic exercise. Vanadium disulfide (VS₂), iron disulfide (FeS₂), and molybdenum diselenide (MoSe₂) decompose hydrogen peroxide (H₂O₂) to generate ROS, corresponding to hydroxyl radicals (•OH), which induce apoptosis and DNA injury in tumor cells (Fig. 5C, D) [169,170,171]. Huang et al. [172] manufactured a multifunctional hydrogel (GMNG), incorporating MoS₂ and gadolinium (Gd)-complex dopants into N-acryloyl glycinamide/Gel-MA (NAGA/Gel-MA), for osteosarcoma therapy. Via its high-efficiency photothermal conversion, MoS₂ eradicated tumor cells each in vitro and in vivo whereas stopping postoperative bacterial infections. This hydrogel system, able to releasing Gd³⁺, concurrently promoted osteogenesis and enabled magnetic resonance imaging (MRI)-based monitoring of its degradation, providing superior methods for bone defect restore (Fig. 5E).
In BTE, 2D TMDs are continuously employed as floor coatings to create a microenvironment conducive to osteoblast development [173]. Moreover, defect-rich MoS₂ nano-assemblies present ample energetic websites, serving as crosslinking facilities that modulate the kinetics and mechanical traits of polymer hydrogels, leading to stronger networks [174]. Ma et al. [175] established an innovated electrospun poly(ε-caprolactone)/MoS₂ (PCL/MoS₂) composite membrane for guided bone regeneration. MoS₂ nanosheets, appearing as each osteogenic promoters and NIR photothermal brokers, provided the composite membrane with excellent photothermal efficiency, stronger mechanical properties and maintained biodegradability. Below NIR irradiation, the PCL/MoS₂ composite membrane considerably boosted the proliferation of BMSCs and aided within the therapeutic of rat tibial lesions in comparison with pure PCL membranes. Yuan et al. [176] proposed a functionalized MoS₂/PDA-arginine-glycine-aspartic acid (RGD) coating for Ti implant. Upon NIR rays, the MoS₂ nanosheets induced localized hyperthermia by way of the photothermal impact, selling speedy glutathione oxidation and heightening bacterial susceptibility to oxidative stress. Concurrently, by means of a ROS-independent oxidative stress mechanism, the MoS₂ compromised bacterial membrane permeability, resulting in protein leakage and adenosine triphosphate (ATP) depletion, successfully inhibiting bacterial proliferation. As well as, the MoS₂ coating raised mobile compatibility and osteogenic exercise by upregulating the expression of osteogenesis-related genes, corresponding to alkaline phosphate (ALP), runt-related transcription issue 2 (Runx2), collagen Kind I (Col I) and osteocalcin (OCN). In vivo research demonstrated that MoS₂/PDA-RGD-modified Ti implants, beneath NIR irradiation, not solely effectively eradicated Staphylococcus aureus (S. aureus) infections but in addition accelerated new bone formation, showcasing exceptional antibacterial and osseointegration-promoting effectivity.
Yu et al. [177] developed a novel biomimetic photothermal nanozyme (MPMP) based mostly on MoS₂, doped with Mg²⁺-modified PDA and coated with amphiphilic polysulfobetaine, for the amelioration of OA. This nanozyme mimicked antioxidant enzymes/hyaluronan synthase, selling cartilage regeneration, enhancing lubrication and scavenging ROS and reactive nitrogen species (RNS) beneath NIR irradiation, thereby mitigating irritation. Analysis exhibits the MPMP nanozyme considerably suppressed the NF-κB/IL-17 signaling pathway whereas activating the MAPK pathway, facilitating cartilage restore and hyaluronic acid manufacturing, finally ameliorating OA signs in a mouse mannequin (Fig. 5F). Furthermore, one other examine highlighted the potential of MoS₂-incorporated polyether ether ketone (PEEK) composites in enhancing synthetic joint supplies. The stable lubricating effectiveness of MoS₂ considerably decreased friction and put on, whereas its layered construction enabled the formation of a steady switch movie beneath shear, decreasing put on charges. MoS₂ additionally improved the compressive energy and hardness of the composite, sustaining wonderful mobile compatibility and selling BMSC proliferation, underscoring its organic benefits in joint prosthetics [178].
Regardless of these promising properties, TMD-based methods proceed to face important challenges in scientific translation. A significant concern lies within the long-term organic security of transition metals, significantly beneath circumstances of power publicity and in metabolically delicate environments like bone marrow. The degradation of TMDs leads to the discharge of steel ions (e.g., M6+, W6+) and the metabolism of sulfur-containing merchandise (e.g., sulfate ions, thiol compounds), resulting in complicated toxicological mechanisms. Yim et al. investigated the degradation pathways of TMDs in mice, reporting that at 3 h post-injection, tungsten dichalcogenide (WS2) confirmed distinct indicators within the liver, spleen, lungs, and kidneys. Three days after intravenous injection, no fluorescent indicators from the nanoparticles had been detected in any of those organs, indicating speedy clearance and decreased long-term toxicity threat. Nevertheless, following intraperitoneal administration, the nanoplates distributed extra quickly to main organs inside 1 h, with hint indicators nonetheless detectable within the liver and lungs at 10 days, suggesting extended retention in comparison with intravenous injection [179]. Xue et al. examined the tissue distribution of MoS2, discovering that pure MoS2 nanosheets collected within the lungs inside 1 h post-administration, whereas indicators within the coronary heart, kidneys, and mind had been minimal. At 1 and a couple of days post-administration, sign depth within the spleen and liver elevated considerably, presumably as a result of measurement of MoS2 particles, as bigger particles could have problem passing by means of the glomerular filtration barrier or crossing vascular limitations within the coronary heart and mind. Thirty days following intravenous injection, a small amount of MoS2 nanosheets remained detectable within the spleen, primarily localized inside the purple pulp area [180]. Totally different TMDs exhibit various in vivo metabolic charges. At some point post-administration, MoS2, WS2, and TiS2 confirmed related accumulation within the liver and spleen, possible attributed to phagocytosis by Kupffer cells and splenic macrophages. Over time, MoS2 exhibited a considerably quicker metabolic price in comparison with WS2 and TiS2, with its in vivo ranges markedly reducing inside one month post-administration. Elevated molybdenum concentrations detected in urine and feces counsel renal and intestinal excretion of MoS2, whereas WS2 and TiS2 show extended retention within the physique. MoS2 degradation primarily yields water-soluble high-valent Mo(VI) oxides, whereas WS2 and TiS2 endure much less full degradation. WS2 shouldn’t be totally oxidized to W(IV)/W(VI) compounds, and oxidized TiS2 types insoluble white TiO2 precipitates [181].
Relating to TMD biocompatibility, exfoliated 2D-MoS2 is mostly thought-about to exhibit low cytotoxicity. Nevertheless, these conclusions predominantly depend on primary cell viability assays and lack complete analysis of cell morphology, metabolism, or transcriptional responses. Some research report conflicting outcomes concerning MoS2 cytotoxicity, doubtlessly as a result of variations in experimental design and materials preparation. Variables together with focus, cell tradition circumstances, cell sorts, publicity parameters, nanosheet construction, and lateral measurement could affect these outcomes. Poisonous results broadly manifest as alterations in cell viability pushed by oxidative stress or mobile internalization. In vivo, MoS2 accumulation in organs can induce irritation and immune responses, contributing to toxicity. Though 3D in vitro cell tradition fashions higher mimic tissue environments for toxicity evaluation, they can’t totally replicate systemic organismal responses [182]. The restricted analysis on TMD toxicity raises issues concerning potential long-term dangers in orthopaedic purposes. Ion launch throughout TMD degradation could set off a “steel ion launch storm.” Extended accumulation of TMD degradation merchandise could trigger organ fibrosis, leading to progressive impairment of hepatic and renal features.
Transferring ahead, translational analysis ought to emphasize the event of biodegradable or ion-doped TMD derivatives that retain therapeutic efficacy whereas decreasing systemic burden. Furthermore, standardized fashions of bone infections, osteosarcoma, and osteoarthritis are wanted to raised simulate scientific circumstances and consider long-term results. Regulatory-oriented research specializing in toxicology, clearance pathways, and immune interactions can be pivotal for enabling scientific adoption.
In abstract, TMDs, with their distinctive construction and multifunctional options, maintain immense promise within the therapy of orthopedic illnesses. Via section engineering, POD exercise and photothermal results, TMDs obtain microbial eradication, tumor ablation and bone tissue regeneration. As analysis progresses, TMDs are anticipated to grow to be key supplies within the growth of progressive therapeutic approaches for orthopedic problems.
(A) The schematic diagram exhibits the preparation of steel 1T-phase MoS2 to semiconductor 2 H-phase MoS2 nanodots from micron-scale 2 H-phase crystals. Amongst them, 1T-MoS2 nanodots can be utilized as a extremely efficient reagent for tumor PTT. Reprinted with permission from Ref [159]. Copyright 2020, John Wiley and Sons. (B) Schematic illustration of steel 2D MoS2 nanosheets obtained by liquid peeling for photothermally enhanced acoustic antimicrobial. Reprinted with permission from Ref [163]. Copyright 2022, Chen et al. (C) Diagrammatic illustration of VS2-PEG preparation and its enhanced most cancers immunotherapy. Reprinted with permission from Ref [169]. Copyright 2023, American Chemical Society. (D) FeS2 nanozymes mixed with most cancers cell-derived exosome remedy results in radiation sensitization by means of an oxidation response, thereby decreasing the radiation dose of radiotherapy. Reprinted with permission from Ref [171]. Copyright 2021, Huang et al. (E) The hydrogel doped with MoS2 nanoroses has the power to kill tumors, forestall bacterial infections and promote the formation of recent bones. Reprinted with permission from Ref [172]. Copyright 2023, John Wiley and Sons. (F) MoS2-based nanozyme-based composites can be utilized for photothermal remedy of osteoarthritis. Reprinted with permission from Ref [177]. Copyright 2023, John Wiley and Sons. 2D, two-dimensional; MoS2, molybdenum disulfide; PTT, photothermal remedy; VS2, vanadium disulfide; PEG, polyethylene glycol; FeS2, iron disulfide
MXenes
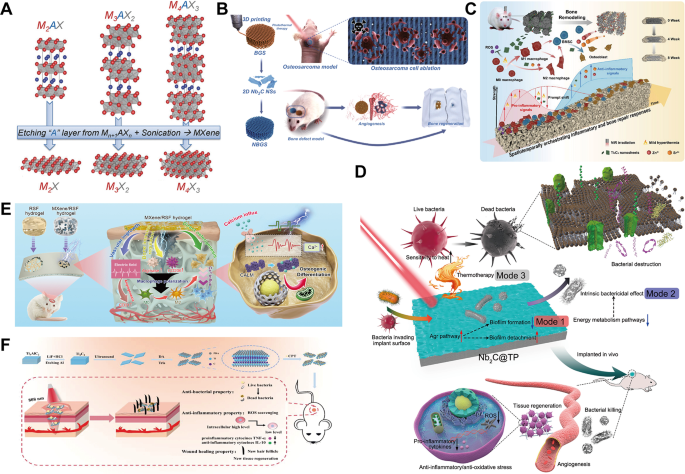
MXenes, a novel class of 2D nanomaterials with a layered topological construction, had been first found in 2011 [183]. These supplies, encompassing transition steel carbides, nitrides or carbonitrides, are represented by the final method Mn+1AXn, the place M denotes an early transition steel, A represents components from teams 13 or 14 of the periodic desk and X stands for carbon or nitrogen (Fig. 6A) [184]. Resulting from their wonderful electrical conductivity, excessive particular floor space, tunable floor purposeful teams and chemical stability, MXenes have garnered important consideration in biomedical purposes.
Among the many degradation merchandise of MXenes, sure transition steel ions are believed to own osteogenic potential. As an illustration, Ti⁴⁺ ions can promote the proliferation and differentiation of osteoblasts, whereas niobium (Nb) ions improve the adhesion and development of those cells (Fig. 6B) [185, 186]. Research have demonstrated that optimum ranges of Ti⁴⁺ can activate inflammatory and osteogenic signaling pathways, such because the PI3K-Akt and ERK pathways (Fig. 6C) [186, 187]. Moreover, each Ti⁴⁺ and Nb, at low concentrations, exhibit antimicrobial exercise, thus decreasing the chance of postoperative infections (Fig. 6D) [188].
Excessive electrical conductivity and biocompatibility of MXenes make them significantly appropriate for supporting osteoblast proliferation and differentiation [189]. This property is particularly advantageous in electrically stimulated bone restore, the place MXenes considerably amplify osteogenic results and enhance bone regeneration [190]. For instance, He et al. [191] synthesized a nanocomposite materials combining MXene with a narrow-bandgap sonosensitizer (VS4) to assemble a Schottky junction-based system (VSM). On this configuration, MXene performed a pivotal position in augmenting the separation effectivity of electron-hole pairs and, by means of its superior conductivity and 2D digital acceptor properties, markedly improved the sonodynamic and chemodynamic results of VS4. This synergy enabled VSM to exhibit excellent antibacterial exercise beneath ultrasonic stimulation whereas enhancing osteogenesis by means of the steady launch of vanadium ions. In one other examine, Hu et al. [192] developed a dual-crosslinked conductive hydrogel community by integrating MXene with regenerated silk fibroin (RSF). This hybrid system successfully transmitted electrical indicators and activated the Ca²⁺/CALM signaling pathway in osteoblasts, thus strengthening their proliferation and differentiation. Furthermore, the hydrogel improved the bone immune microenvironment and facilitated angiogenesis, underscoring its distinctive worth as a conductive materials for bone restore purposes (Fig. 6E).
Ti₃C₂Tₓ, a consultant MXene materials, sometimes carries floor purposeful teams (Tₓ) corresponding to -OH, -F, or -O, which come up from its publicity to acidic or alkaline options throughout synthesis [184]. These surface-active websites elevate the water solubility and dispersibility of Ti₃C₂Tₓ whereas inhibiting bacterial development by means of oxidative stress, making it a promising antimicrobial coating for orthopedic implants. Yu et al. [193] demonstrated that the in situ oxidation of Ti₃C₂, pushed by CaO₂-mediated H₂O₂ launch, generated TiO₂ sonosensitizers and catalytically energetic Ti³⁺. This course of considerably boosted ROS manufacturing, thereby elevating antibacterial effectivity. Concurrently, the discharge of Ca²⁺ ions facilitated mineralization on the bone defect web site, supporting bone regeneration. Each in vitro and in vivo experiments revealed that composite nanosheets exhibited distinctive antibacterial and bone restore capabilities in prosthetic joint an infection and osteomyelitis fashions. Moreover, MXenes can act as carriers for antibiotics or antimicrobial peptides, enabling the managed launch of those brokers and increasing their antimicrobial efficacy (Fig. 6F) [194].
MXene supplies, corresponding to Ti₃C₂Tₓ, additionally exhibit notable photothermal results beneath NIR irradiation, straight damaging or inducing apoptosis in tumor cells [195]. Yin et al. [185] developed a 3D-printed bone-mimicking scaffold (NBGS) incorporating 2D niobium carbide (Nb₂C) MXene nanosheets. This scaffold achieved twin performance for osteosarcoma remedy and bone defect restore. Nb₂C MXene exhibited wonderful photon responsiveness within the second NIR window, effectively ablating osteosarcoma cells by means of photothermal results. Concurrently, the degradation of the scaffold launched Nb-based substances, selling angiogenesis and delivering oxygen and vitamins to assist bone restore. Moreover, the discharge of calcium and phosphate ions throughout degradation accelerated new bone tissue mineralization. This multifunctional method, integrating tumor photothermal ablation, angiogenesis promotion and osteogenesis, highlighted immense potential of MXenes in osteosarcoma therapy and BTE. Constructing on this, Yang et al. [196] launched a mesoporous construction into related NBGS to allow exact and managed nitric oxide (NO) launch. Gradual launch of low-concentration NO considerably promoted angiogenesis, demonstrating an clever and adjustable biomaterial platform.
In conclusion, MXenes signify an rising class of multifunctional 2D nanomaterials that supply exceptional benefits for orthopedic purposes, together with excessive electrical conductivity, photothermal responsiveness, antimicrobial exercise and osteoinductive potential. Their skill to modulate bone regeneration, eradicate pathogens and facilitate tumor ablation positions them as promising candidates for the following era of good bone implants and therapeutic scaffolds.
(A) The construction of the MAX section and the corresponding MXenes obtained by chemically selectively etching the A layer. Reprinted with permission from Ref [184]. Copyright 2014, John Wiley and Sons. (B) The mixing of Nb2C into BG can be utilized for photothermal-driven osteosarcoma ablation remedy with potent angiogenesis and osteogenic results. Reprinted with permission from Ref [185]. Copyright 2021, Yin et al. (C) Diagrammatic illustration of a ceramic bracket adorned with Ti3C2Tx. Promotes bone therapeutic by channeling dynamic irritation and bone tissue responses. Reprinted with permission from Ref [186]. Copyright 2024, Huang et al. (D) Schematic Nb2C@TP of multimodal an infection management biofunctions with bacterial clearance and tissue regeneration capabilities. Reprinted with permission from Ref [188]. Copyright 2021, American Chemical Society. (E) MXene loaded electroactive hydrogels promote environment friendly bone regeneration in an electro-microenvironment. Reprinted with permission from Ref [192]. Copyright 2022, Hu et al. (F) Schematic diagram of the synthesis of MXene-based antimicrobial nanosystems, presenting promising antimicrobial and anti inflammatory methods. Reprinted with permission from Ref [194]. Copyright 2023, John Wiley and Sons. Nb2C, niobium carbide; TP, titanium plate
Others
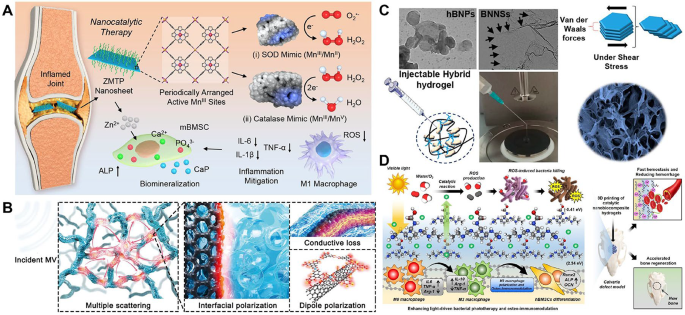
Within the exploration of superior supplies for orthopedic purposes, rising candidates corresponding to metal-organic frameworks (MOFs), hBN and g-C3N4 provide immense potential regardless of their comparatively nascent stage of utility on this area. These supplies display distinctive physicochemical properties, together with distinctive tunability, mechanical resilience and bioactivity, making them promising for addressing complicated challenges in bone restore, osteogenesis and antibacterial remedy. Nonetheless, present analysis predominantly focuses on their basic properties and preliminary biomedical explorations, highlighting the necessity for complete preclinical research to completely unlock their translational potential in scientific settings.
MOFs
MOFs, composed of steel ions or clusters coordinated with natural ligands, exhibit extremely tunable porosity, in depth particular floor areas and memorable chemical and thermal stability. These porous constructions allow environment friendly loading of a variety of osteogenic, anti-tumor medicine in addition to bioactive molecules, corresponding to plasmids, non-coding RNAs, exosomes or autophagy activators, making them promising candidates for treating illnesses like OA, bone defects, avascular necrosis and osteosarcoma [197,198,199,200,201,202]. Two-dimensional steel–natural frameworks (2D MOFs) possess the next particular floor space, and their ultrathin thickness maximizes publicity of surface-active steel websites, making them extremely amenable to modification. Consequently, they’re more and more favored in orthopaedic purposes. Qiao et al. developed a medium-pressure response system based mostly on an interface-limited 2D MOF grown on carbon nanotubes (CNTs). The ultra-small copper-based 2D MOF exhibited ample floor and interface defects, endowing the system with quite a few floor states (Fig. 7B). Below microwave (MV) irradiation, the CNT-2D MOF successfully absorbed microwaves and transformed them into warmth by way of enhanced heterointerface polarization, enabling microwave thermal remedy. Concurrently, floor states facilitated era of excited electrons for microwave dynamic remedy. This CNT-2D MOF system demonstrated extremely environment friendly broad-spectrum antibacterial exercise towards seven pathogenic micro organism, together with each Gram-negative and Gram-positive strains, after solely 7 min of MV irradiation. Cell viability and morphology assays following co-cultivation with CNT-2D MOF revealed no important variations in comparison with controls. Though rising focus barely decreased cell viability, even at 1 mg/mL viability remained above 94%. Hemolysis assays with rabbit purple blood cells confirmed no important hemolysis, with solely a 6.3% hemolysis price noticed at 1 mg/mL. Moreover, liver and kidney operate indicators in vivo confirmed no important variations between CNT-2D MOF-treated teams and controls, suggesting favorable in vivo security and environment friendly clearance. In a rabbit osteomyelitis mannequin, microwave therapy raised native temperature from 28 °C to 42 °C inside 7 min, demonstrating good in vivo thermal stability. After 5 days of therapy, Staphylococcus aureus counts in bone marrow decreased dramatically to 0.619 × 10 CFU/g in comparison with 7.181 × 10 CFU/g in controls, evidencing potent antibacterial efficacy [203].
Given the complicated pathophysiological setting in orthopaedic illnesses, the efficiency, in vivo stability, and degradation kinetics of 2D MOFs are important. As an illustration, the native inflammatory milieu in arthritis could have an effect on MOF stability and performance. Shi et al. synthesized 2D manganese porphyrin-based MOF nanosheets (ZMTP) by way of coordination of MnTCPP + with Zn²⁺ benzoate oxygen teams, utilizing them as antioxidant nanoenzymes mimicking superoxide dismutase (SOD) and catalase actions (Fig. 7A). Step potential shift measurements in electrolytes indicated excessive redox stability. Lengthy-term degradation assessed by dispersing ZMTP in weakly acidic PBS revealed a two-stage degradation: preliminary small pore formation after 12 h, progressive pore enlargement over 24 h, and near-complete degradation by 96 h, indicating preliminary upkeep of catalytic exercise adopted by degradation and clearance guaranteeing biosafety. In an arthritis mouse mannequin, ZMTP therapy considerably downregulated pro-inflammatory cytokines in joints and upregulated alkaline phosphatase (ALP) ranges, demonstrating efficient anti-arthritis and enhanced osteogenic outcomes. Histological analyses of main organs (coronary heart, liver, spleen, kidneys) confirmed negligible pathological modifications, confirming good in vivo clearance [204].
Bone defects following tumour resection typically resist self-repair and threat tumour recurrence or an infection, necessitating antibacterial bone fillers with sturdy in vivo stability. Sure 2D MOFs excel as a result of their ultrathin layers, excessive floor space, and in depth π-conjugated rings, which allow speedy response to exterior gentle, excessive photothermal conversion effectivity, and wonderful stability. Wu et al. fabricated 2D cobalt-coordinated tetra(4-carboxyphenyl)porphyrin (Co-TCPP) MOF nanosheets exhibiting ultrathin thickness and enormous floor space, facilitating swift gentle responsiveness and environment friendly photothermal conversion. The π-conjugated porphyrin macrocycles confer exceptional photothermal stability. Included into calcium phosphate cement (CPC), 2D Co-TCPP MOF accelerated CPC degradation, possible as a result of comparatively excessive dissolution price of Co-TCPP, with out compromising photothermal efficiency. Below laser irradiation, the 1% Co-TCPP/CPC composite quickly elevated temperature, sustaining above 50 °C, leading to important tumour necrosis discount. The injectable paste conformed nicely to bone defect morphology. Over an 8-week interval, CPC degraded on the defect web site whereas selling in depth new bone formation and neoangiogenesis. These outcomes point out that 2D MOF nanosheets keep the intrinsic properties of CPC, with steady degradation and photothermal efficiency conducive to orthopaedic tumour therapy [205].
h-BN
hBN, sometimes called white graphene, is a 2D crystalline nanomaterial composed of boron and nitrogen atoms organized in a hexagonal honeycomb lattice and its physicochemical properties in addition to structural traits bear a hanging resemblance to these of graphite crystals [206]. The exceptional mixture of excessive tensile energy and distinctive chemical inertness has facilitated its in depth exploration inside the medical area. At current, hBN nanosheets are predominantly being engineered for superior bioimaging purposes and drug supply methods, significantly in oncological therapeutics.
Regardless of its immense potential, analysis into the utilization of hBN for orthopedic problems stays comparatively nascent. Experimental proof has elucidated that the incorporation of hBN may markedly increase the mechanical robustness of composite supplies, thereby enabling the fabrication of superior bone-regenerative scaffolds [207, 208]. As an illustration, Ensoylu et al. [209] efficiently built-in hBN nanoparticles (0.1%−2%) into borate-based BG scaffolds, observing a pronounced enhancement in compressive energy and in vitro mineralization capabilities. Concurrently, drug launch research revealed that hBN expedited the discharge kinetics of gentamicin and 5-fluorouracil, adhering to first-order and Higuchi fashions respectively. As well as, the antibacterial attributes of hBN render it exceptionally promising for mitigating bone an infection dangers. Mukheem et al. [210] embedded hBN inside a polyhydroxyalkanoate/chitosan (PHA/Ch) polymeric matrix leveraging its thermal stability and nanoscale morphology. This refined nanocomposite exhibited potent bactericidal efficacy towards MRSA and multidrug-resistant E. coli K1 strains, whereas sustaining wonderful cytocompatibility with negligible cytotoxicity. These findings underscore its viability as a foundational component within the growth of antibacterial scaffolds for biomedical purposes.
Analysis by Goncu et al. illuminated the profound affect of hBN on the rheological conduct of hydrogel matrices. The intrinsic lubricity of hBN allowed its uniform dispersion inside the hydrogel community, considerably diminishing zero-shear viscosity and deformation resistance. Such rheological refinements spotlight that hBN enhances the dynamic synergy of hydrogels, providing compelling theoretical underpinnings for its potential deployment in joint lubrication and OA therapeutics (Fig. 7E) [211].
The applying of hexagonal boron nitride (hBN) in orthopaedics stays at an early stage, with a number of challenges requiring consideration to realize constant dispersion inside composite scaffolds. The intrinsic chemical inertness and hydrophobicity of hBN nanoparticles hinder their steady dispersion in aqueous or biocompatible solvents. Furthermore, robust van der Waals interactions amongst hBN nanoparticles typically result in agglomeration, leading to uneven distribution inside the scaffold and localized stress focus. Manufacturing processes additional affect hBN dispersion; for example, shear forces throughout 3D printing can promote nanoparticle aggregation, whereas ice crystal formation throughout freeze-drying could compress hBN particles, inflicting localized enrichment zones. Subsequently, optimizing dispersion strategies is crucial, together with floor functionalisation approaches or the event of efficient and steady dispersants and stabilizers. Composite dispersion methods, corresponding to combining mechanical mixing with ultrasonic therapy, will also be employed to boost uniformity throughout scaffold fabrication.
Relating to biocompatibility, Bianco et al. reported that hBN exerts decrease toxicity on dendritic cell (DC) exercise and DC-mediated T cell polarization in comparison with MoS₂ and graphene oxide (GO) [212]. Some research combining hBN with osteoblast-like cells noticed decreased cell viability, doubtlessly as a result of nanoparticle internalization triggering reactive oxygen species (ROS) era. Conversely, different analysis discovered no cytotoxic results at hBN concentrations under 22 µg/mL, with indications of decreased oxidative stress. At low concentrations, hBN doesn’t induce mitochondrial depolarization or disrupt F-actin cytoskeleton formation [213]. However, investigations into the mobile compatibility of hBN stay restricted. Crucially, the long-term results of boron launched from hBN, together with native bone tissue accumulation and systemic distribution, in addition to security thresholds, require additional elucidation. The biodegradation mechanisms and metabolic pathways of hBN are but to be totally understood. Inefficient clearance of nanoparticles could provoke power inflammatory responses and organ injury. Moreover, present research predominantly deal with in vitro assays or short-term in vivo experiments. Important knowledge on the long-term in vivo conduct of hBN—corresponding to biocompatibility, particle launch kinetics, power irritation, and organ accumulation—stay inadequate and warrant complete investigation.
g-C3N4
g-C3N4 is an rising visible-light-responsive non-metallic semiconductor materials. Davardoostmanesh et al. [214] demonstrated that g-C3N4 nanosheets, ready by way of electrophoretic measurement fractionation, exhibit distinctive photoluminescence properties. In comparison with bulk g-C3N4, these nanosheets show considerably larger cytotoxicity towards human osteosarcoma cell line Saos-2, whereas exhibiting negligible toxicity to regular human foreskin fibroblasts. Equally, Taheri et al. [215] reported that photoactivated g-C3N4 can successfully induce most cancers cell loss of life with out the necessity for extra photosensitizers or chemotherapeutic brokers, as it’s effectively internalized by tumor cells in vitro. The underlying mechanism includes the activation of pathways related to oxidative stress, cell loss of life and apoptosis, with its anticancer impact being strictly light-dependent. For regular cells, g-C3N4 exhibits negligible cytotoxicity. That is attributed to the selective accumulation of photosensitizers in tumour tissue mixed with exact spatial management of sunshine publicity, which ensures that ROS era happens primarily inside tumour cells or their microenvironment. Given their extraordinarily quick lifespan and restricted motion vary, ROS cytotoxicity is strictly confined to areas with concentrated photosensitizers beneath gentle publicity. Furthermore, tumour cells exist in a state of oxidative stress, rendering them extra inclined to ROS-induced injury, thereby enhancing the selective cytotoxicity in the direction of tumour cells. Nevertheless, as a result of ROS lack intrinsic specificity, misdirected supply of g-C3N4 to wholesome tissues or improper management of sunshine publicity could lead to injury to wholesome bone tissue. Subsequently, additional analysis is critical to boost focused supply of photosensitizers and obtain exact management of sunshine publicity, thereby minimizing ROS-induced injury to wholesome tissues.
Within the context of osteogenesis, g-C3N4 has additionally demonstrated exceptional potential. Sadek et al. [216] revealed that g-C3N4 upregulates osteogenic marker genes corresponding to Col I, OCN and osteopontin in vitro and considerably enhances bone therapeutic in a rabbit critical-sized femoral condyle defect mannequin. That is evidenced by larger percentages of bone-like tissue, elevated collagen maturation and improved biodegradability. Moreover, Dutta et al. [217] discovered that oxidized g-C3N4 (Ox-gCN), when included into alginate/gelatin hydrogel scaffolds, considerably boosts the adhesion, proliferation and osteogenic differentiation of hBMSCs. That is achieved by means of the enrichment of oxygen-containing purposeful teams on its floor and the activation of ERK and MAPK signaling pathways, which additional promote macrophage M2 polarization and the expression of anti-inflammatory genes, thereby facilitating bone immunomodulation. Furthermore, its visible-light-induced photocatalytic properties effectively generate ROS, inhibiting bacterial biofilm formation and stopping an infection. In a rat calvarial defect mannequin, the Ox-gCN scaffold expressed superior hemostatic, anti-inflammatory and bone regeneration capabilities, establishing itself as a multifunctional platform for the restore of traumatic bone accidents (Fig. 7F).
Regardless of these developments, the scientific translation of those supplies nonetheless faces important challenges. Though biodegradable supplies have been explored in experimental settings, their in vivo security and long-term results stay insufficiently investigated. Furthermore, the degradation kinetics of those supplies in animal fashions are inadequately characterised, leading to important data gaps. Moreover, regulatory pathways and standardized analysis methods for these supplies are underdeveloped, contributing to extended timelines for scientific translation.
From a producing perspective, manufacturing typically includes complicated solvothermal circumstances and incurs excessive prices. Scale-up processes face challenges corresponding to poor batch-to-batch consistency and elevated purification bills. Even slight variations in manufacturing parameters can considerably have an effect on materials efficiency. Moreover, the conduct of those supplies within the complicated pathophysiological setting of the human physique should be rigorously thought-about. Affected person-specific components, together with age, metabolic illnesses, and genetic background, could affect material-host interactions, thereby affecting the reproducibility and efficacy of scientific purposes. Overcoming these translational bottlenecks necessitates multidisciplinary collaboration. Future analysis ought to deal with methods corresponding to floor functionalization to boost biocompatibility and biodegradability, alongside rigorous and complete toxicity evaluations—significantly inside immunocompromised and bone defect fashions—to assist safer and simpler scientific translation.
There may be additionally a necessity for modular integration of those supplies into composite platforms—corresponding to 3D-printed scaffolds, injectable methods, and responsive coatings—to enhance their scientific usability. Interdisciplinary collaboration can be essential to unlock the total potential of those supplies, linking advances in nanomaterial science with orthopedic surgical procedure, microbiology, and tissue engineering. With additional mechanistic elucidation and translational design, these rising supplies are nicely positioned to complement and even transcend typical bone substitutes, significantly in complicated instances involving an infection, irritation, or tumor recurrence.
(A) Schematic diagram of the mechanism of motion of ZMTP nanosheets within the therapy of rheumatoid arthritis utilizing nanocatalysis. Mimics the exercise of superoxide dismutase (SOD) and catalase, downregulates oxidative stress, and promotes osteogenesis. Reproduced with permission from Ref [204]. Copyright 2022, Springer Nature. (B) Schematic diagram of the microwave absorption mechanism of CNT-CuHHTP. Displays antibacterial therapeutic results in osteomyelitis. Reprinted with permission from Ref [203]. Copyright 2023, John Wiley and Sons. (C) hBN added to hydrogels can scale back the dynamic viscosity and deformation price of polymers with zero shear factors and is anticipated to be a drug candidate for the therapy of osteoarthritis. Reprinted with permission from Ref [211]. Copyright 2023, American Chemical Society. (D) Ox-gCN-modified hydrogel scaffolds obtain anti-inflammatory and bone immunomodulation for traumatic bone damage restore. Reproduced with permission from Ref [217]. Copyright 2024, Elsevier. MOF, metal-organic framework; β-TCP, beta-tricalcium phosphate; ROS, reactive oxygen species; Ox-gCN, oxidized g-C3N4