Biochemical properties of dECM
One of many main benefits of decellularized extracellular matrix (dECM) is its potential to facilitate structural reworking by supporting the formation of particular tissues on the implantation website, moderately than leading to functionally insufficient scar tissue [29]. Current research have proven that bio-scaffolds generated from tissue-specific matrices can improve performance [30] and complicated tissue formation [31], highlighting the advantages of tissue specificity. For these causes, we chosen porcine dECM because the reconstruction materials for neural tissue following intracerebral hemorrhage (ICH). The preparation of dECM and bioprinting supplies was carried out following bodily and chemical processing, as illustrated in Fig. 1A.
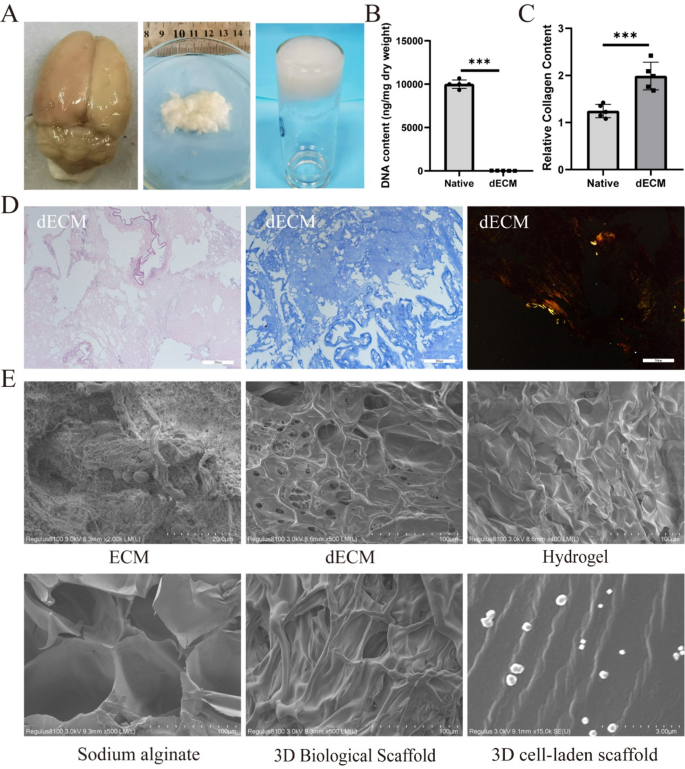
Preparation and characterization of 3D cell-laden scaffold. (A) Pictures of decellularized mind matrix and hydrogel preparation. (B) Quantitative evaluation of DNA content material (n = 4). (C) Quantification of collagen retention fee (n = 4). (D) Hematoxylin and eosin (HE) staining and Masson staining demonstrating decellularization, with Sirius Purple staining confirming collagen fiber retention. (E) Scanning electron microscopy (SEM) evaluation of ECM, dECM, hydrogel, sodium alginate, 3D organic scaffold, and 3D cell-laden scaffold. Scale bars: D 200 μm/100 µm, E 100 μm/3 µm. Information had been expressed as imply ± commonplace deviation. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
It’s effectively established that residual mobile supplies inside ECM can set off immune responses as soon as implanted right into a recipient’s physique [32]. If the nuclear materials throughout the matrix is lowered to low ranges (i.e., DNA content material under 50 ng/mg of dry tissue weight) [33], it not solely avoids eliciting any immune response however might also stimulate constructive tissue reworking [33]. To evaluate the efficacy of decellularization, we analyzed the DNA and collagen content material in each native and decellularized tissues. The evaluation confirmed that greater than 99% of DNA was efficiently faraway from dECM (Fig. 1B), and the collagen content material in dECM surpassed that of contemporary neural tissue (Fig. 1C). HE staining additional confirmed the absence of residual mobile materials following decellularization (Fig. 1D).
The decellularization course of successfully eliminates mobile constituents whereas preserving important extracellular matrix (ECM) structural proteins, together with collagen. Enhanced collagen retention in decellularized tissues not solely reinforces scaffold mechanical integrity but in addition establishes a biomimetic microenvironment conducive to mobile adhesion and migration. Comparative evaluation revealed that hUCMSCs cultured on ECM-coated scaffolds exhibited 3.1-fold greater adhesion effectivity (p < 0.001) in comparison with these on uncoated controls (Determine S1A). Morphologically, hUCMSCs on ECM-coated substrates displayed well-spread phenotypes with in depth pseudopodia formation, whereas cells on naked plates remained predominantly rounded and exhibited minimal attachment. These findings show that collagen and related ECM parts retained post-decellularization critically mediate cell-matrix interactions, thereby potentiating adhesion dynamics.
Subsequent, we evaluated the composition of dECM, as one other problem throughout decellularization is discovering a steadiness between efficient removing of mobile parts and retention of ECM materials. Collagen fibers are vital constituents of the basement membrane [34], and their presence additional ensures the profitable retention of key ECM parts in the course of the decellularization course of. An intact basement membrane performs an important position in tissue development and differentiation, significantly in tissue engineering [35]. Masson staining and Sirius Purple staining had been carried out to look at collagen in dECM. Ends in Fig. 1D point out that the composition of dECM intently resembles that of native tissue, which can guarantee the advantages of using dECM-based bioink. Moreover, SEM evaluation revealed that there have been no residual mobile parts in dECM, and the orientation and construction of those fibers remained largely unaffected by the decellularization course of. Our findings recommend that dECM is appropriate to be used in 3D cell-laden scaffold. Though SEM confirmed that dECM, hydrogel, and sodium alginate had been randomly organized with out efficient spatial construction, 3D bioprinting yielded bio-scaffolds with organized preparations that possess outlined spatial structure. We included hUCMSCs into the bioink to synthesize 3D cell-laden scaffold by way of 3D printing, and SEM photographs displayed an orderly association and uniform distribution (Fig. 1E).
Collagen fibers represent a elementary structural ingredient of the basement membrane [34], and their preservation is pivotal for retaining important extracellular matrix (ECM) parts throughout decellularization. In native cerebral tissue, the basement membrane represents solely 5–10% of whole ECM (primarily localized to perivascular areas and meninges), whereas the interstitial matrix, dominated by kind I collagen fibrils, accounts for > 60% of the ECM mass [34]. The structural integrity of the basement membrane is indispensable for orchestrating tissue growth and mobile differentiation, significantly in tissue engineering functions requiring biomimetic microenvironmental reconstitution [35]. To safeguard important ECM constituents (laminin, heparan sulfate proteoglycans), we employed a delicate decellularization technique combining 0.5% Triton-X100 with nuclease digestion. Though kind I collagen predominated within the 3D cell-laden scaffold, strategic retention of basement membrane parts.
Characterization of bioink
The era of 3D cell-laden scaffold includes a number of steps together with dECM extraction, bioink preparation, sterilization, cell encapsulation, and 3D printing (Scheme 1). Amongst these, bioink formulation is important. A notable limitation of dECM is its poor mechanical properties. Earlier research have utilized brain-cell matrix hydrogels mixed with silk fibroin to print 3D organic scaffolds [28], however silk fibroin possesses higher stiffness than brain-cell matrix hydrogels. Mismatches in mechanical properties between printed constructions and pure tissues can result in issues. Due to this fact, decrease stiffness supplies ought to be chosen to interchange silk fibroin, or acceptable methods ought to be employed to match mechanical properties.
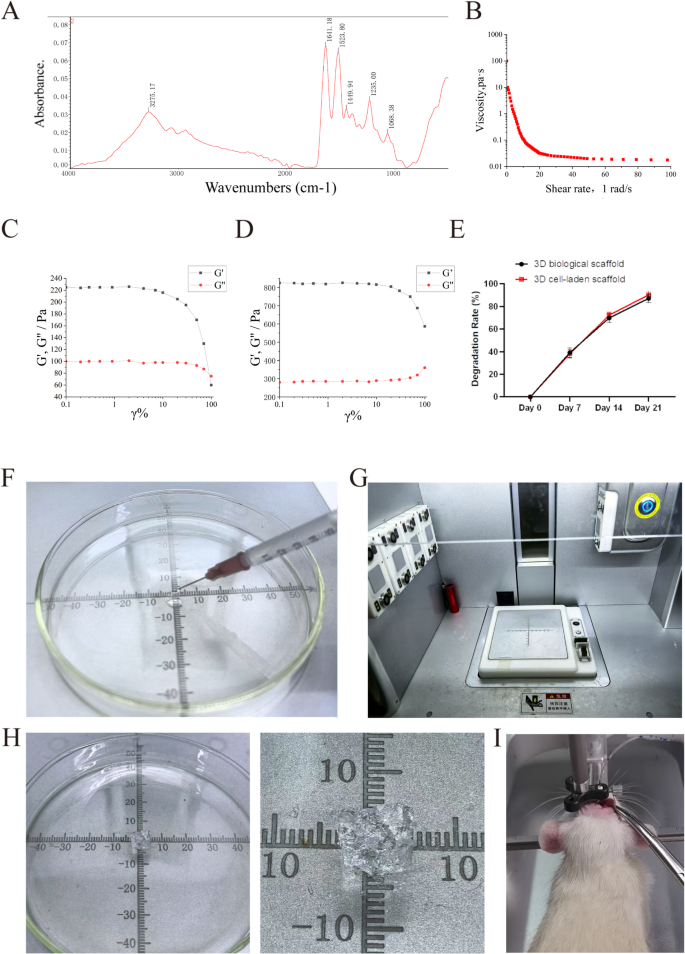
Because of this, we mixed dECM hydrogels with sodium alginate to create a bioink. The dECM hydrogel and collagen are homologous [36], exhibiting excessive viscosity and ease of freezing. Within the presence of divalent cations (equivalent to Ca²⁺ and Sr²⁺), alginate can chemically cross-link with them to boost the hydrogel’s stiffness. Scanning electron microscopy (SEM) revealed that the general microstructure of the bioink was a porous community (Fig. 1E). Fourier-transform infrared spectroscopy (FTIR) displayed attribute alerts of the bioink, together with C-N at 1633.59 cm⁻¹ and NH₂ at 1537.14 cm⁻¹, indicating a big presence of positively charged teams throughout the bioink (Fig. 2A). Moreover, as shear charges elevated, viscosity regularly decreased, confirming the bioink’s injectability (Fig. 2B and F). The rheological properties of the dECM hydrogel and bioink are illustrated in Fig. 2C and D. The G′ values for each dECM hydrogel and bioink considerably exceeded their G″ values, demonstrating solid-like conduct. The G′ worth of the bioink (roughly 800 Pa) was considerably higher than that of the dECM hydrogel (roughly 200 Pa), indicating a big enhancement in mechanical properties by means of calcium ion cross-linking whereas sustaining a extremely porous 3D construction, thereby permitting nutrient diffusion to help ongoing reconstruction. We included hUCMSCs into the bioink and synthesized 3D cell-laden scaffold by way of 3D bioprinting (Fig. 2G and H), which had been subsequently transplanted in situ into rats following ICH (Fig. 2I).
Preparation and characterization of 3D cell-laden scaffold. (A) FTIR evaluation of the 3D organic scaffold bioink, exhibiting a peak at 1537 cm⁻¹ equivalent to the NH₂ group, and C = O stretching detected at 1633 cm⁻¹. (B) Variation in viscosity of the bioink because the shear fee will increase from 1 s⁻¹ to 100 s⁻¹. (C) Rheological properties measured to guage the mechanical efficiency of decellularized mind matrix (dECM) bioink. (D) Rheological properties measured to evaluate the mechanical efficiency of the 3D organic scaffold bioink. (E) Degradation fee of 3D organic scaffold and 3D cell-laden scaffold (n = 4). (F) The 3D organic scaffold reveals injectability. (G) 3D bioprinter. (H) Preparation of 3D cell-laden scaffold. (I) In situ transplantation of 3D cell-laden scaffold. Information had been expressed as imply ± commonplace deviation. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
Alginate, a pure polysaccharide derived from seaweed, is extensively utilized in bioinks as a consequence of its wonderful biocompatibility and printability [12]. Nonetheless, at excessive concentrations (10% w/v), alginate varieties a dense hydrogel community that limits cell motion and spreading. The excessive crosslinking density of alginate creates a inflexible matrix, lowering the area out there for cells emigrate. Alginate lacks intrinsic cell-binding websites (RGD sequences), that are important for cell adhesion and motility [17, 18]. In our experiments, the restricted spreading of hUCMSCs throughout the 3D-printed scaffold is attributed to the excessive alginate focus. Whereas this restricts cell motility, it additionally supplies mechanical stability to the scaffold, which is essential for sustaining structural integrity throughout printing and post-printing tradition.
The scaffold stiffness was optimized to 1.8 ± 0.3 kPa (by way of lowered crosslinking from 30 to fifteen min) to keep away from exceeding the two kPa threshold for pathological glial activation, which triggers astrocyte hypertrophy [22]. This modulus adjustment suppressed gliosis (GFAP⁺ cells at 3.0 kPa). Residing throughout the regenerative stiffness window (1–2 kPa) for human iPSC-derived neural progenitors [23], the scaffold facilitated neuropil-mimetic synaptogenesis and directional axon steerage. Future designs incorporating matrix metalloproteinase-responsive hydrogels [24] may dynamically modulate stiffness to emulate developmental neurogenic softening, doubtlessly augmenting endogenous restore mechanisms.
Biocompatibility of bioink
Alginate dissolves quickly by means of ion change (Ca²⁺ launch), whereas ECM collagen degrades slowly by way of enzymatic hydrolysis, synergistically regulating the degradation fee. The reasonable degradation fee (60% in 14 days) ensures short-term scaffold stability whereas regularly releasing ECM-derived restore alerts (Fig. 2E).
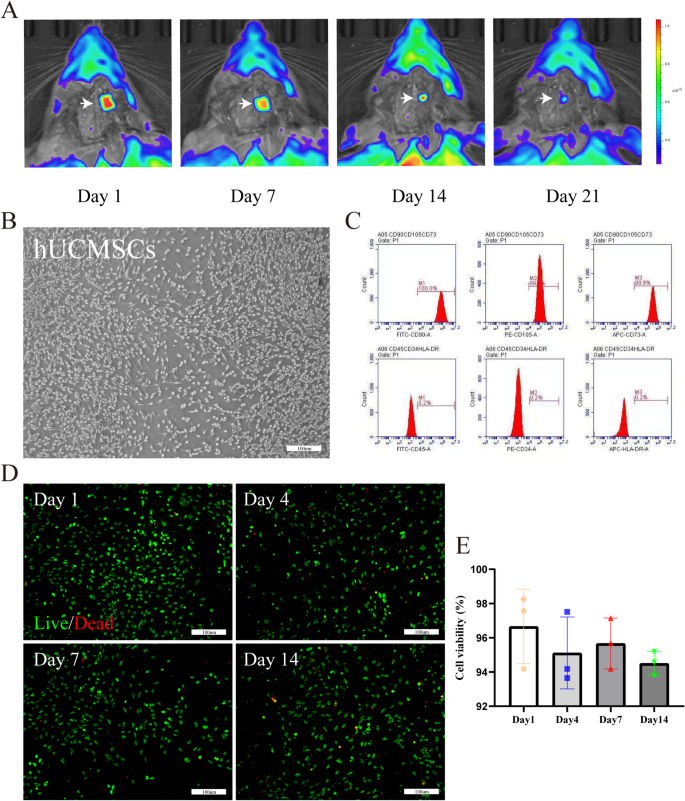
The bioink used for 3D cell-laden scaffold should be secure and biocompatible [37]. Longitudinal in vivo monitoring of scaffold biodegradation was carried out utilizing a luciferase-labeled 3D cell-laden scaffold implanted stereotaxically into the cerebral hemorrhage area of Sprague-Dawley rats (n = 4). Bioluminescence imaging (IVIS® Spectrum, PerkinElmer) at 0, 7, 14, 21 days post-implantation revealed a 92.3 ± 5.1% sign depth discount by day 21 (Fig. 3A).
Analysis of 3D cell-laden scaffold. (A) In vivo biodistribution and degradation of luciferase-labeled 3D cell-laden scaffold. (B) Optical evaluation of hUCMSCs. (C) Move cytometry evaluation confirmed hUCMSCs categorical CD73, CD90, and CD105 however not CD34 and CD45. (D) 1–14 days to evaluat viability of hUCMSCs throughout the 3D cell-laden scaffold utilizing an in vitro reside/lifeless assay. hUCMSCs had been derived from passage 3 (P3), exhibiting inexperienced fluorescence (reside cells with calcein AM) and purple fluorescence (lifeless cells with EthD-1) as noticed beneath a laser confocal microscope. (E) Quantitative evaluation of the cell viabilities (n = 3). Scale bars: B, D 100 μm. Information had been expressed as imply ± commonplace deviation. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
Viability of hUCMSCs in 3D organic scaffolds in vitro
As proven in Fig. 3B, hUCMSCs from passage 3 (P3) adhered and grew, exhibiting a fibroblast-like morphology. Move cytometry confirmed that hUCMSCs remoted from human umbilical twine expressed stem cell-specific marker proteins equivalent to CD73, CD90, and CD105, however didn’t categorical hematopoietic marker proteins equivalent to CD34 and CD45 (Fig. 3C). 3D cell-laden scaffold can differentiate into neuronal cells (NEUN was optimistic, Determine S2A), glial cells (GFAP was optimistic, Determine S2B), endothelial cells (CD31 was optimistic, Determine S2C) and so forth beneath particular induction.
The shear forces utilized throughout printing may doubtlessly harm the cells throughout the printed constructions. Moreover, cytotoxic residues from the dECM bioink could have an effect on cell viability. Due to this fact, we assessed the survival of hUCMSCs throughout the 3D organic scaffold utilizing a reside/lifeless assay (Fig. 3D). At day one, hUCMSCs displayed a excessive viability (> 95%) throughout the scaffold. Remarkably, after fourteen days, cell viability remained excessive (Fig. 3E). Much like earlier research [28], the 3D organic scaffold maintained its unique 3D microstructure previous to degradation, offering substrates for cell embedding and establishing an optimum surroundings for cell survival. Cells transplanted into the 3D organic scaffold can secrete matrix proteins to ascertain their very own ECM regionally, thus reaching extra refined tissue reworking upon shedding the scaffold’s bodily help. The excessive survival fee of hUCMSCs throughout the 3D organic scaffold after one week of in vitro tradition signifies that this scaffold can present limitless vitamins and oxygen to the cells embedded inside, facilitating long-term tradition.
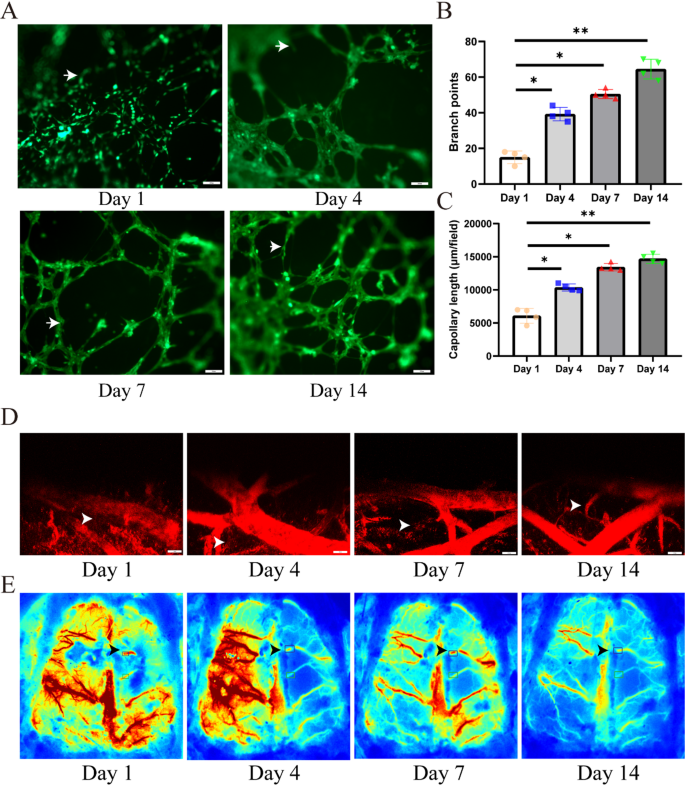
As depicted in Fig. 4A, we evaluated the impact of the 3D organic scaffold on vascularization of P3 hUCMSCs. Outcomes demonstrated a big improve within the lumen community fashioned by hUCMSCs over time (indicated with white arrows), with a 40% improve in vascular density (p < 0.05). The structural integrity of the vascular formations was confirmed, with bigger lumen diameters noticed at day fourteen (Fig. 4C), alongside a considerably greater frequency of lumen formation in comparison with the day seven (Fig. 4B). These findings spotlight the important position of the 3D organic scaffold in selling neovascularization in the course of the vascularization means of hUCMSCs.
Analysis of 3D cell-laden scaffold. (A) In vitro tube formation assay demonstrating the affect of hUCMSCs on vascular reconstruction throughout the 3D cell-laden scaffold. (B, C) Consider the branche and capollary size of the reconstructed vessels (n = 4). (D, E) Cerebral hemodynamic parameters, together with regional cerebral blood circulate (rCBF) and vascular permeability, had been quantitatively evaluated peri-hematomally utilizing laser speckle distinction imaging (LSCI) and dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) at postoperative days 1, 4, 7, and 14. Scale bars: A 200 μm, D 100 μm. Information had been expressed as imply ± commonplace deviation. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
The 3D cell-laden scaffold considerably enhanced perihematomal vascular regeneration and restored distal blood circulate perfusion (as assessed by laser speckle distinction imaging; Fig. 4D and E). Over time, the 3D cell-laden scaffold demonstrated superior neovascularization, indicating their optimistic position in selling angiogenesis.
It’s noteworthy that the formation of vascular tissue throughout the implanted construction is a key discovering of our in vivo research. We suggest that hUCMSCs mixed with the bioink exert a synergistic impact on the angiogenesis of 3D cell-laden scaffold. Analysis has indicated that hUCMSCs can facilitate tissue regeneration by means of angiogenesis [24, 25]; subsequently, it’s believable that the bioink could induce angiogenesis from the host’s surrounding tissues across the implant.
In vivo analysis of 3D printed 3D cell-laden scaffold
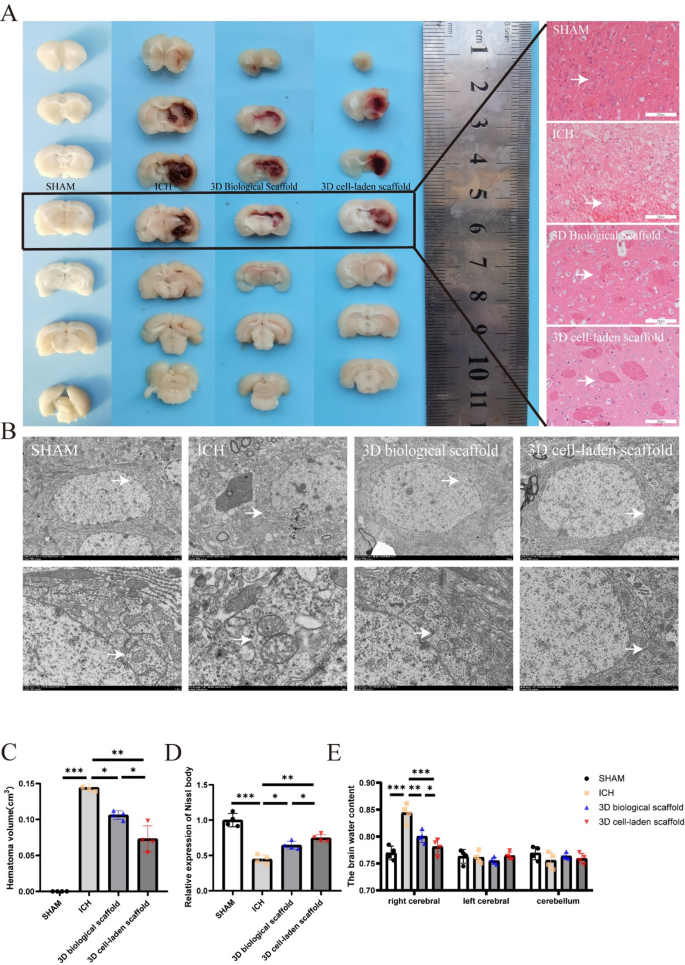
In vivo research confirmed the profitable engraftment of 3D organic scaffolds and 3D cell-laden scaffold following ICH induction in rats. Histopathological evaluation demonstrated a 51.2% discount in hematomal quantity (p < 0.01) within the 3D cell-laden scaffold group versus each management and acellular scaffold cohorts (Fig. 5A and C). Hematoxylin-eosin (H&E) staining revealed attenuated vascular dilation and diminished perivascular inflammatory infiltrates within the 3D cell-laden scaffold group (Fig. 5A). Ultrastructural examination by way of transmission electron microscopy (TEM) confirmed preserved mitochondrial structure (cristae integrity: 82.4% vs. 48.7% in controls) and endothelial continuity in 3D cell-laden scaffold-treated tissues, with minimal proof of membrane rupture or inflammatory extravasation (white arrows, Fig. 5B).
In vivo analysis of 3D cell-laden scaffold. (A) Consultant photographs of hemorrhagic lesions in rats from every group, stained with hematoxylin and eosin (HE). (B) Transmission electron microscopy (TEM) evaluation of rats from every group, evaluating mitochondrial cristae loss, outer membrane rupture, and vascular dilation (indicated by white arrows). (C) Quantity of intracerebral hemorrhage in rats from every group (n = 4). (D) Nissl staining of mind tissues from rats in every group (n = 4). (E) Evaluation of mind water content material in rats from every group to guage the impression of remedy on cerebral edema (n = 4). Scale bars: A 400 μm/100 µm, B 4 nm/1 nm. Information had been expressed as imply ± commonplace deviation. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
Nissl staining quantification indicated a 1.5-fold improve in viable neurons inside perihematomal areas of the 3D cell-laden scaffold group in comparison with controls (p < 0.01), accompanied by lowered necrotic particles (Fig. 5D). Cerebral edema evaluation at 5 days post-ICH confirmed a 24.6% lower in mind water content material (p < 0.001), confirming enhanced neuroprotection (Fig. 5E).
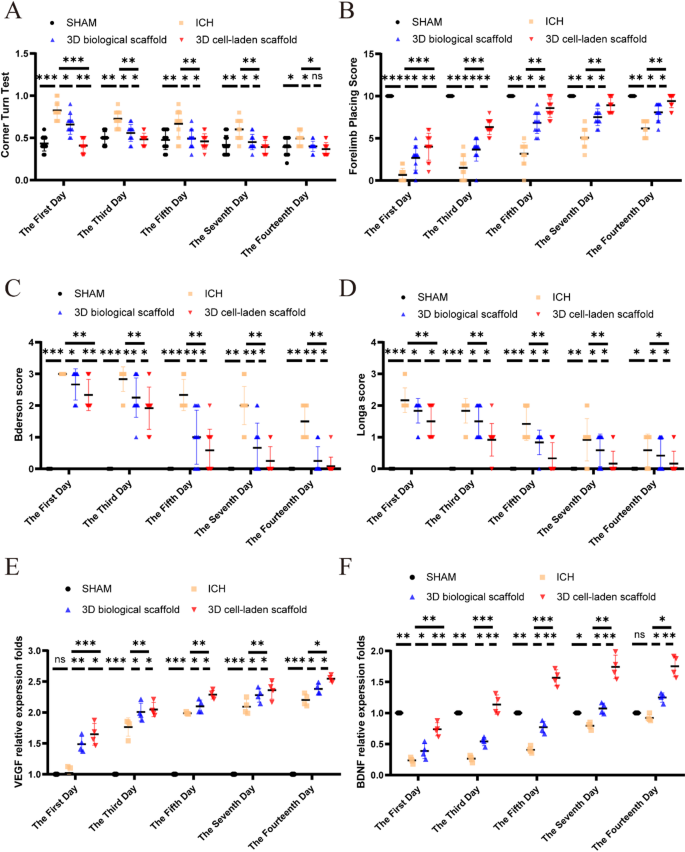
Neurobehavioral assessments carried out at days 1, 3, 5, 7 and 14 post-transplantation quantitatively evaluated the impression of 3D cell-laden scaffold on neurological harm. Sensorimotor capabilities had been assessed utilizing forelimb placement and rotation assessments, whereas total neurological operate was evaluated utilizing Longa and Bederson scoring techniques. In comparison with the hemorrhagic group, remedy with 3D cell-laden scaffold considerably enhanced the efficiency of rats within the turning check (Fig. 6A) and improved accuracy within the forelimb placement check (Fig. 6B). Neurological operate scores considerably declined 24 h after ICH; nevertheless, following remedy with 3D cell-laden scaffold, there was a notable enchancment in neurological operate (Fig. 6C and D).
In vivo analysis of 3D cell-laden scaffold. (A) Nook check carried out from days 1 to 14 post-intracerebral hemorrhage (ICH) (n = 12). (B) Forelimb placement check outcomes (n = 12). (C) Bederson rating and (D) Longa rating had been utilized to evaluate neurological restoration (n = 12). (E, F) Statistical evaluation of VEGF and BDNF mRNA (n = 4). Information had been expressed as imply ± commonplace deviation. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
The impact of 3D cell-laden scaffold on neurological restore was quantitatively assessed at 1, 3, 5, 7, and 14 days after transplantation. To quantitatively analyze the mRNA expression ranges of VEGF and BDNF at totally different time factors after ICH utilizing qPCR, examine their dynamic adjustments in relation to neurological restoration, and validate the regulatory results of 3D cell-laden scaffold on angiogenesis and neurotrophic signaling pathways. 3D cell-laden scaffold upregulated VEGF to advertise angiogenesis and enhance perfusion in ischemic areas (Fig. 6E) and upregulated BDNF to inhibited neuronal apoptosis and enhanced synaptic plasticity (Fig. 6F).
Differentiation of CM-Dil labeled hUCMSCs in 3D cell-laden scaffold
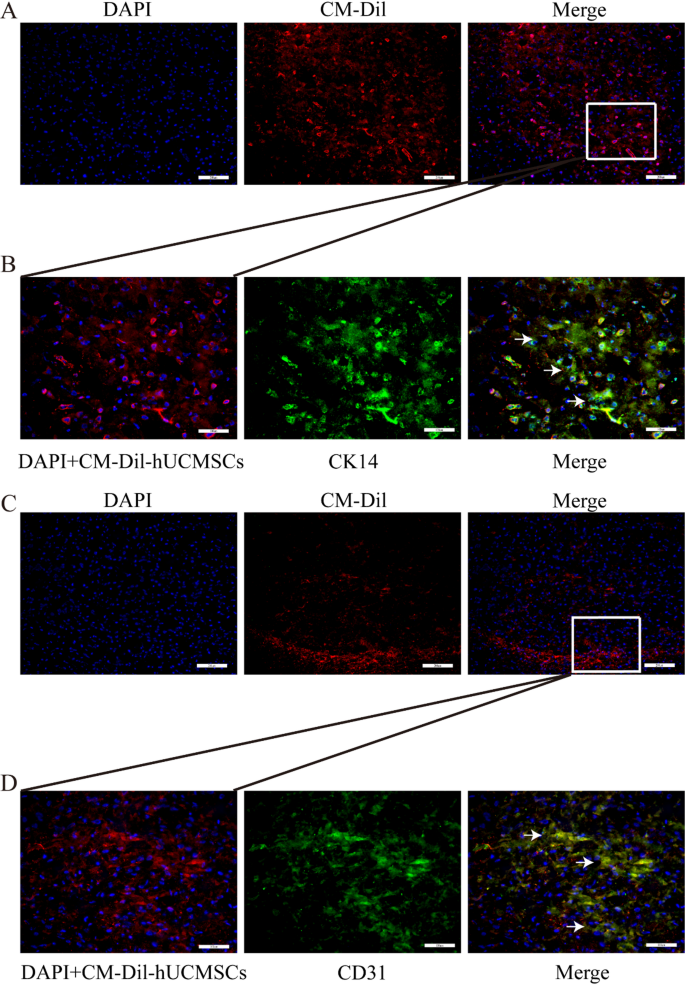
hUCMSCs are a sort of stem cell recognized for his or her potential to reside inside grafts with out present process transformation. Underneath particular induction, it could actually differentiate into neuron cells (NEUN optimistic, Determine S2A), glial cells (GFAP optimistic, Determine S2B), endothelial cells (CD31 optimistic, Determine S2C), and so on. Quite a few experimental experiences point out that stem cells can differentiate into epithelial lineage cells, equivalent to urothelial cells [38] and airway epithelial cells [39]. Consequently, we investigated the traits of hUCMSCs post-implantation. DAPI was used to stain the cell nuclei, showing blue, whereas CM-Dil was localized throughout the cell membranes and cytoplasm, exhibiting a purple coloration. Consultant frozen sections stained with DAPI confirmed that hUCMSCs remained viable in vivo at day fourteen post-implantation (Fig. 7A and C). Goal proteins appeared inexperienced, whereas the mixture of inexperienced, purple, and blue resulted in a white overlay. We noticed some co-expressed loci localized inside keratinocytes (CK14 optimistic, Fig. 7B) and endothelial cells (CD31 optimistic, Fig. 7D), according to different experiences [20,21,22,23,24,25,26], demonstrating that hUCMSCs co-printed with bioink to synthesize 3D cell-laden scaffold acquired epithelial-like and endothelial-like phenotypes submit in situ transplantation. This will, to some extent, clarify why vascularization and epithelialization in 3D cell-laden scaffold generated extra optimistic alerts in comparison with the 3D organic scaffold group.
Differentiation of CM-Dil-labeled hUCMSCs inside 3D cell-laden scaffold. (A, C) Viability of hUCMSCs in 3D cell-laden scaffold at week 4 post-transplantation. (B, D) Expression of CK14 and CD31 throughout the 3D cell-laden scaffold constructed with encapsulated hUCMSCs, assessed at 4 weeks post-transplantation. Scale bars: A-D 200 μm